We’re talking 60-plus — women and men. And we’re talking about hitting the gym and weight training. Don’t be put off, Bamman says.
“Resistance training is in many ways the true fountain of youth,” Bamman said in an interview with The Associated Press. “I like to say the fountain of youth is the water cooler in the gym.”
Of course, there are biological limits. But Bamman says the bulk of age-related decline in strength, flexibility and endurance is behavioral — putting too few demands on the body, not too many.
“When I tell somebody that in four to six months your strength and muscle mass and overall muscle function is going to elevate to the levels of people 30 to 35 years younger, that hits home,” he said.
So you know you’re too sedentary and the birthdays keep piling up. You suspect resistance training would be beneficial. But perhaps you’re intimidated. Don’t be.
Getting started
Check with medical professionals to make sure there are no health problems that stand in your way.
Then find a gym. Larger gyms offer a social component with things to do on a day off from weight training. And Bamman suggests getting a trainer.
“It’s actually quite safe, but it does require proper progression,” Bamman said. “You have to have a good instructor who can teach the movements properly.”
Bamman, a research scientist at the Florida Institute for Human & Machine Cognition, said finding a fully qualified trainer can be tricky.
“We need more rigorous certification of trainers,” he said. “The problem is that you can go online tonight and pay $50 and get some certification as a trainer.”
Done and dusted in an hour
Bamman suggests resistance training twice a week. Three times is even better, and he recommends non-weight-training days in between. For instance, work out on Monday, Wednesday and Friday, and let Tuesday and Thursday be days of rest.
He suggests 10 different exercise movements — eight is sufficient. Do 10 repetitions of each movement. Do this three times, described as three sets. Then move on to the next movement.
When you reach the 10th repetition, you should feel you can’t do many more. If you could have done 10 more reps, you might want to increase the resistance.
Bamman says machines are better for beginners, but free weights — barbells or dumbbells — may be more effective as you gain confidence.
Before the weights, start with a 5-10 minute warmup — on the treadmill, stationary bike or elliptical machine — to get the blood flowing. You can add a few minutes on the mat for stretching and abdominal work.
Then come the weights.
“Sometimes you see people who sit on the machine, do a set and then play with their phone for three or four minutes. We like to keep them moving.”
Women may benefit even more than men
Women may benefit from resistance training even more than men because it’s a way to fight osteoporosis, the loss of bone density.
“Women are on a disadvantaged trajectory for bone loss, particularly in susceptible areas such as the hips and lower back,” said Bamman, who completed his doctorate at the University of Florida College of Medicine.
“But the strength-training benefits for both sexes are really important. There are no sex differences in the ability to respond. In gaining muscle mass and strength in untrained people, men and women track the same.
Yasuko Kuroi is 72 and started resistance training about 20 years ago.
“I saw the men in the gym and thought I could do that, too,” she said, speaking at a municipal recreation center in Tokyo.
The body demands work
In a few words: Use it or lose it.
Bamman cautions against pampering yourself and criticized even health care professionals “who baby seniors.” Of course, common sense is required.
“Our human body is a demand-based system,” he explained. “If you chronically impart a low demand on the body, we have adaptations to low demand. That’s why we lose muscle mass, that’s why we get weaker. We’re not demanding much.”
“But if you put high demands on the system — like resistance training — now the body has to adapt to these higher demands. The body says: ‘To adapt to these new demands I’ve got to make my bones stronger. I’ve got to make my muscles bigger.”
Bamman used the example of space flight, or extended bed rest, where people rapidly lose strength.
“Bed rest or space flight is essentially expedited aging,” he said. “All of our systems as we age are capable of responding and adapting. They just need the stimulus.” He said he’s seen positive effects for people in their 70s and 80s, and even for some in their 90s.
Bamman is 57 and joked he’s getting “closer in age to the people I study.” He also emphasized there are no shortcuts.
“These programs that roll out for older adults — seated exercises and the like. This is gimmicky and they don’t impart enough demands on the body,” he said.
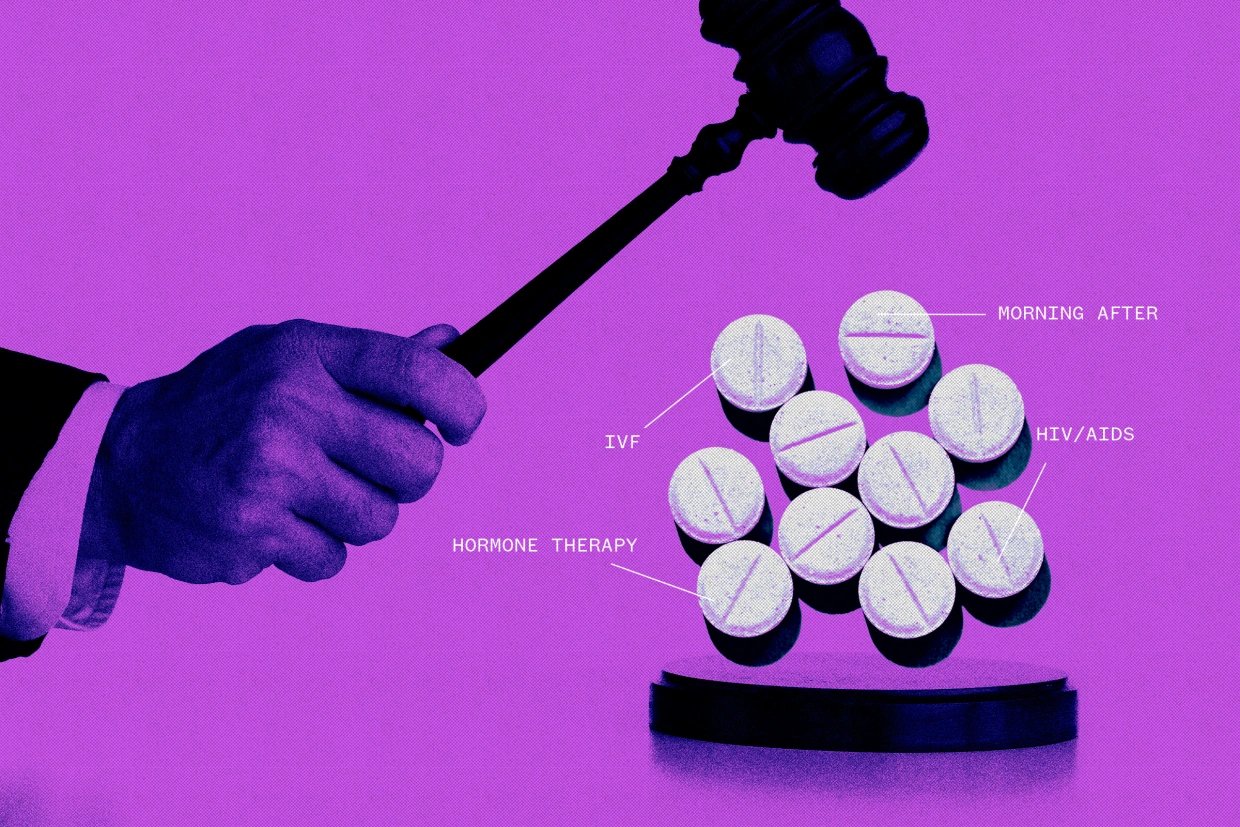
]]>The ruling from the 5th U.S. Circuit Court of Appeals is narrow, applying only to the eight employers who objected to providing the coverage. The conservative court declined to make the ruling apply nationwide.
“While we were predicting the worst, at the moment insurers will still have to cover preventive services, including PrEP, except for the original plaintiffs. That is the good news,” Carl Schmid, executive director of the HIV+Hepatitis Policy Institute, said in an email, referring to a common HIV preventative treatment. But, Schmid lamented that the court found that the coverage requirement for HIV prevention was adopted in violation of the Constitution, and that the case is going back to a lower court for resolution of other issues that could further muddy the coverage issue.
The requirements in question were adopted by federal health officials under provisions of the Affordable Care Act, sometimes referred to as Obamacare. Challengers raised religious and procedural objections to some of the requirements.
U.S. District Judge Reed O’Connor in Texas ruled last year that the requirements violated the Constitution. In its ruling Friday, a three-judge 5th Circuit panel said the coverage requirements in question were adopted unconstitutionally because they came from a body — the United States Preventive Services Task Force — whose members were not nominated by the president and confirmed by the Senate.
Not all preventive care is threatened by the ruling and attorneys on both sides said that some employers could decide to adopt copays or deductibles that would keep the affected coverages, including HIV preventatives, available, if not free.
An analysis prepared last year by the KFF, a nonprofit, found that some screenings, including mammography and cervical cancer screening, would still be covered without out-of-pocket costs because the task force recommended them before the health care law was enacted in March 2010.
Meanwhile, the opinion left some issues unresolved, including whether coverage can be required that was adopted from recommendations by two other entities, the Public Health Service’s Advisory Committee on Immunization Practices, and the Health Resources and Services Administration.
“The bad news is, the court still finds the mandate to cover USPSTF recommended services unconstitutional and now asks the lower court to review both the HRSA and ACIP preventive services,” Schmid said.
The U.S. Department of Health and Human Services did not immediately respond to an emailed request for comment Friday afternoon.
]]>“This is absurd,” Sanders said in an interview late Tuesday. “It is clear that Novo Nordisk is ripping off the American people.”
Novo Nordisk CEO Lars Fruergaard Jørgensen has agreed to testify in September over the pricing of the drugmaker’s hugely popular weight loss drugs, the Senate Committee on Health, Education, Labor and Pensions announced Friday.
It came three days after Sanders, who chairs the committee, threatened to hold a vote to subpoena Novo Nordisk President Doug Langa to provide testimony.
In a separate statement, a spokesperson for Novo Nordisk said that Jørgensen agreed after he and Sanders had “a productive call and agreed to find a mutually acceptable date for a hearing.”
Sanders said Tuesday that he is still working with Novo Nordisk to set an exact date for the hearing, but he expects it will be during the second week of September. He said his strategy for getting Novo Nordisk to reduce the cost of the drugs is simple: Put the company in the spotlight.
“I think enough public pressure may result in them lowering their prices substantially, which is obviously what my goal is,” the senator said. “This is a huge issue because it is likely that Ozempic and Wegovy may end up being the most lucrative product that the pharmaceutical industry has ever developed.”
In April, the committee launched an investigation into Novo Nordisk’s pricing practices. It cited a report that found that Novo Nordisk charges around $1,300 a month for Wegovy in the U.S., even though the drug can be purchased for $186 a month in Denmark, $137 in Germany and $92 in the United Kingdom. Some patients in the U.S. have said that the higher prices of Ozempic and Wegovy have pushed them to unregulated, copycat drugs for weight loss.
In reality, there’s little Congress can do about the high cost of the weight loss drugs in the U.S. Novo Nordisk charging higher prices is partly a function of how the American patent system works.
Drugmakers who develop new medications get to exclusively sell them on the U.S. market for a set period of time — typically 20 years. During this time, other companies can’t make generic versions of the drug, severely limiting competition. And unlike other countries, there is generally no direct pricing regulation on the drug by the federal government.
The patent for semaglutide, the active ingredient in Ozempic and Wegovy, isn’t expected to expire until around 2031 at the earliest.
When asked about prescription drug pricing in the U.S., Sanders responded, “This is the system, it’s a corrupt system.”
“It’s a system controlled by a large pharmaceutical industry, who makes massive amounts of campaign contributions,” he said. “And it’s a system which enables the drug companies to make huge profits, while 1 out of 4 Americans cannot afford to buy the prescription drugs that doctors prescribe.”
Sanders said he knows Novo Nordisk likely isn’t the only one responsible for the high cost of the drugs. He said so-called pharmacy benefit managers, also known as middlemen, likely also make the cost of the drugs more expensive for people in the U.S.
Sanders said Novo Nordisk had wanted other companies, including pharmacy benefit managers, on the panel in September, but he declined.
“None of that negates the fact that they are charging us far, far more for the exact same product than they charge people in other countries,” he said.
Novo Nordisk declined to provide an additional comment on Sanders’ remarks.
This article first appeared on NBCNews.com. Read more from NBC News here:
]]>Some people are turning to supplements to manage those issues rather than using treatments approved by the Food and Drug Administration, such as hormone therapy. But, experts say, menopause supplements aren’t necessarily helpful. And, in some cases, they can be dangerous.
The rising popularity of menopause supplements, experts say, highlights a lack of access to evidence-based options, rampant misinformation about hormone therapy and gaps in our knowledge about menopause — even among specialists.
Current menopause treatments
“The primary treatment — and the first-line treatment — should be hormone (estrogen) therapy, especially for moderate-to-severe menopause symptoms,” Dr. Anna Barbieri, assistant clinical professor in the department of obstetrics and gynecology at Mount Sinai School of Medicine, tells TODAY.com
“There is just nothing as effective and nothing that has as wide-ranging effects and benefits as hormone therapy,” Barbieri adds.
Effectively managing hot flashes, most often through hormone therapy, may have long-term health benefits, too, Dr. Lauren Streicher, medical director of the Northwestern Medicine Center for Sexual Medicine and Menopause, tells TODAY.com.
Not only do hot flashes last seven years on average (and often longer for Black women), but we also know that “hot flashes are associated with cardiovascular disease, brain fog during perimenopause, potentially declines in cognitive function down the road and multiple other medical problems,” Streicher says.
However, some people should steer clear of hormone therapy due to other health conditions. That includes a current or past hormone receptor-positive cancer (primarily breast and endometrial cancers), as well as a history of stroke, blood clots or cardiovascular disease, Barbieri explains.
If someone can’t or prefers not to take hormone therapy, that’s where non-hormonal options come in. Those include the off-label use of antidepressant medications and the recently-approved drug fezolinetant.
There are also all kinds of lifestyle and behavior techniques people may use to stay generally healthy, whether or not those techniques directly help with hot flashes, Dr. Monica Christmas, associate professor and director of the Center for Women’s Integrated Health at The University of Chicago Medicine, tells TODAY.com.
That might include avoiding certain triggers (such as alcohol), cognitive behavioral therapy, maintaining good diet and exercise habits and even hypnosis, she says.
What doctors don’t want is for you to jump into taking supplements on your own.
“I cannot tell you how many times I have identified supplements people were using that where unnecessary, potentially harmful or interacting with other medications,” Barbieri says. “Or people who were using 25 supplements where they could just use hormone therapy.”
Should you take menopause supplements?
There are a lot of reasons someone might want to try an over-the-counter supplement to manage menopause symptoms. Of course, there’s a wealth of options available on the internet — many with little or no evidence that they actually work, and some with evidence that they can be harmful.
“Most of them just haven’t been studied,” Streicher says. “But some of them we know absolutely are not safe to use.”
In its 2023 position statement on non-hormone therapies, the Menopause Society (formerly known as the North American Menopause Society) did not recommend any dietary supplement to manage menopause symptoms. For the majority of the supplements the group looked at, their conclusions were due to flawed studies, mixed results or an overall lack of evidence.
The experts TODAY.com spoke to generally agree, but take a more nuanced position: There can be a place for supplements in managing menopause symptoms, they say. But their usefulness depends on your symptoms, what other treatments you’re comfortable with and the specific supplements you’re using.
For Streicher, it makes sense to talk about supplements when patients have only mild symptoms, or if they have more intense symptoms and already take a prescription medication but want to try something on top of that.
A typical example for Barbieri might be a patient with breast cancer and sleep issues who can’t take hormone therapy. “If someone does not use medication for sleep, and lifestyle interventions don’t work, then we may turn to something like magnesium and l-theanine or inositol,” she says, which research shows are safe for patients with a history of breast cancer who cannot take hormone therapy.
Other people “feel that menopause is natural … and they just want to use certain lifestyle interventions and only feel comfortable with supplements no matter what,” Barbieri says. “That’s OK, I just need to know that.”
More than anything, experts say, the decision whether to take menopause supplements should be based on a knowledgeable provider having a genuine conversation with a patient about what really might work for them and their preferences. (edited)
“As providers, we do need to be able to give good information and understand that (supplements are) going to be a preference of some people,” Christmas says. “And if it doesn’t work, (we need to be) respectful that maybe shoving hormone therapy down their throat still might not be the answer.”
Common ingredients in menopause supplements
There are a ton of supplements on the market, many of which contain proprietary blends of ingredients.
Additionally, supplements are not regulated by the FDA in the same way that pharmaceutical drugs are. It’s up to the supplement manufacturer and distributors to ensure the safety and correct labeling of their products, the FDA explains.
That’s why, if you’re going to use any supplements, the experts recommend looking for a label that indicates it has been third-party verified, which means you can be more confident that it actually contains the ingredients that it advertises. Specifically, Barbieri suggests looking for USP or GMP supplement certifications on a product.
And keep the placebo effect in mind, Christmas says. When you take something new, you might feel better initially. “But the placebo effect can’t be sustained,” Streicher says.
Here are a few common ingredients you may see in menopause supplements:
Vitamins and minerals
If someone is deficient in vitamins and minerals during menopause, supplements may be a good idea, experts say.
For instance, low levels of vitamin D are correlated with poor bone health and depression, Barbieri explains. “Because these are factors that are important for all of my menopausal patients, I will actually check vitamin D and will replace that — no question,” she says.
The same goes for vitamin B12 and ferritin (a form of iron) which women may not get enough of via food, particularly those following plant-based or vegetarian diets. “These are essential nutrients that are going to result in improved health and sense of well-being if someone is deficient in them,” Barbieri says.
If you think you could benefit from a supplement to treat a deficiency, talk to your doctor about testing first.
Isoflavones, phytoestrogens and “plant-based hormones”
Phytoestrogens are plant compounds that mimic estrogen when broken down in the body. Isoflavones are a type of phytoestrogen that comes from soybean products.
This is a major category of menopause supplements, but they are not safe for people avoiding hormone therapy due to their health histories, the experts say.
“If you have a history of an estrogen-derived cancer, like a breast cancer, you shouldn’t be taking (phytoestrogen supplements) either,” Christmas says. “Those are nuances that maybe people don’t think about when they see a supplement in the health food store or local pharmacy,” she adds.
For those interested in phytoestrogen supplements who have a low-risk health history, Streicher recommends looking into S-equol. “It’s the only one that really has science (behind it),” she says, “because it’s the active metabolite of soy, which is what’s been shown to actually potentially help with hot flashes.”
Just keep in mind that people’s experiences with these kinds of supplements vary widely, Streicher says, because people metabolize them differently.
While phytoestrogen supplements are not recommended for people avoiding hormone therapy, those patients generally are OK to eat phytoestrogens occurring naturally in food, such as soy, Barbieri explains.
What about general “plant-based hormone” products? Creams are often marketed with such language, Barbieri says, adding that they may contain a version of progesterone made in a lab using a compound in yams, she says. That language is aimed at people who want to feel natural by using that, but “plants do not have human hormones,” she stresses.
Black cohosh
“Black cohosh is commonly reported to help with hot flashes and night sweats, although it hasn’t been proven to do that,” Christmas says, “and it actually can increase liver enzymes.”
If you have an underlying liver condition, you should steer clear of black cohosh she says. Or, if you decide to take it, “you should be monitored frequently with serology to make sure your liver function isn’t worsening,” Christmas adds.
St. Johns wort
Often advertised to help with hot flashes and depression, St. Johns wort can also interact with many other medications that can have an impact on someone’s health, Christmas says.
Combinations of herbs
Some products contain a huge blend of many types of botanical ingredients and may or may not actually list the ingredients, Streicher says. For instance, you don’t know what you’re getting with products labeled simply “Chinese herbs,” she says.
Before you try menopause supplements…
It’s tempting to just buy the bottle, but the experts discouraged trying a new supplement without talking to your doctor.
First, set up a doctor’s appointment just to talk about menopause. Resist the urge to simply tack a conversation about menopause onto the end of another appointment, Christmas says.
Making an appointment specifically for menopause symptoms “automatically sets a different tone,” she explains, and “it’s probably going to get you a little bit more time in front of that health care professional.” Ideally, this should be an in-depth and individualized discussion to go through your specific symptoms, what you’ve already tried and your treatment preferences.
Unfortunately, many doctors — even OB-GYN specialists — don’t receive much (if any) training in menopause, Streicher says.
So, if your doctor isn’t well-versed in menopause treatments, find a specialist. All the experts recommend starting by using this tool from the Menopause Society to find a menopause expert near you.
If you’re in a major city, you’ll likely be able to find someone pretty easily, Streicher says. But if not, telehealth consultation services like Midi and Gennev may be your best option. (Streicher provides educational work for Midi.)
This story first appeared on TODAY.com. More from Today:
]]>- A record number of mosquitoes are testing positive for West Nile virus in and around Las Vegas amid a surge in the area’s overall mosquito population.
- Local health officials are urging the public to take precautions to avoid getting bit.
- As climate change expands the reach of insect-borne diseases, Las Vegas’ situation is offering a case study.
A record-breaking number of mosquitoes in and around Las Vegas are carrying West Nile virus, sparking warnings from local health officials who say the public should take precautions to avoid getting bit.
West Nile virus can cause fever, headaches, vomiting and diarrhea and is fatal in about 1 of 150 cases. There are no vaccines or medications to treat or prevent the mosquito-borne illness.
In recent weeks, 169 of over 24,000 pools of mosquitoes tested for West Nile virus returned positive — meaning at least one insect in the pool carried the disease — across 25 southern Nevada ZIP codes. The number of mosquitoes recorded and the tally of positive pools this early in the season break the area’s records for both metrics, set in 2019.
“These are huge numbers of mosquitoes, and we’ve already identified a concerning number of them carrying the West Nile virus,” said Vivek Raman, an environmental health supervisor for the Southern Nevada Health District.
Health officials have also identified six pools in the Las Vegas area that tested positive for St. Louis encephalitis virus, a mosquito-borne disease that can cause fatal inflammation of the brain.
For decades, climate scientists and public health officials have warned that climate change could expand the reach of various infectious diseases, especially those spread by mosquitoes. Las Vegas’ exploding mosquito population and the local uptick in West Nile prevalence offers an important case study on how climate could affect human health.
Climate change increases average global temperatures and precipitation levels, fostering conditions that are ideal for mosquitoes, which breed in still, warm water. It also extends the length of warm periods, prolonging the active season for mosquitoes. These changes increase the risk of human exposure to diseases like West Nile virus, even in places that have never recorded cases before.
The first case of West Nile virus in Las Vegas was recorded in 2004 — five years after the United States’ first case was documented in 1999 in New York City. Las Vegas’ most recent West Nile outbreak occurred five years ago, resulting in 43 human cases. District health officials are concerned that this summer could be far worse.
In Nevada and much of the Southwest, springtime weather has become warmer and summertime heat waves have grown increasingly extreme over the last few decades. Las Vegas has seen average springtime temperatures rise by 6.2 F since 1970; this month, the city has already experienced a weeklong, record-breaking heat wave.
Southern Nevada’s rising temperatures are creating favorable conditions for mosquitoes, said Nischay Mishra, an assistant professor of epidemiology at Columbia University. What’s more, ongoing drought conditions in the state, which have led to low water table levels throughout the Colorado River Basin, including in Lake Mead, may also be counterintuitively beneficial for the insects.
“Mosquitoes typically thrive in wet and hot places,” Mishra said. “But in Nevada, as smaller bodies of water dry up, they create shallow waters that are ideal for mosquito breeding.”
Las Vegas’ mosquito surge has been giant: Last year, district health officials measured 6,000 mosquitos in traps across Clark County from April to June. This year, counts have already exceeded 24,000.
The vast majority have been Culex mosquitoes, a primary vector for West Nile virus. But another mosquito species that does not carry the virus, Aedes aegypti, has also become more common in Las Vegas. Aedes was first spotted in the area in 2017, and Raman attributes its spread there to the impacts of climate change, as well.
Along with climate, human behavior plays an important role in the spread of vector-borne diseases. Aedes and Culex mosquitoes both thrive in the backyards of many Las Vegas homes — the former breed in small pools of water such as those left from sprinklers, while the latter often breed along the surface of unmaintained swimming pools.
Raman said the best way to avoid infection is to empty any open containers filled with water outside, maintain swimming pools, wear protective clothing and use bug spray to avoid getting bit.
Louise Ivers, a professor of global health and social medicine at Harvard Medical School and the director of its Global Health Institute, said situations like the one in Las Vegas will become more common as climate change continues to boost infectious disease globally.
“We should expect to see new infectious diseases, old infectious diseases back again and a change in the patterns of exposure of existing infectious diseases like West Nile virus,” Ivers said. “Things that we used to do freely without worrying as much about protection from vectors like mosquitoes or ticks, we might not be able to do anymore.”
This story first appeared on NBCNews.com. More from NBC News:
]]>“I gotta stick around. I want to stay alive for the next 40 years: be a grandpa, see my kids’ kids, take them to the gym, tell them the stories of when I was in the NBA around ‘Bron and Kob’ and all these guys,” he told Men’s Health magazine in an interview that published Tuesday. “I gotta fight for it.”
Robinson’s kidneys have been functioning at less than 15 percent of their optimal capacity, according to the magazine. He said some days after getting dialysis, a procedure to remove waste and excess fluid from the blood after the kidneys stop working properly, he gets body cramps, shortness of breath, and experiences violent vomiting.
On days when he feels good after treatment, he trains at the gym and spends time with his children. He said having a routine has helped him get out of what he calls “the dark place.”
“I was so angry in the beginning,” he told the publication. “I was so mean.”
The former Chicago Bulls guard played 11 seasons in the NBA for eight different teams, including the Nuggets, Thunder, Pelicans, Knicks, Warriors, Celtics, Clippers and Bulls. He made a name for himself in New York when he became the first player in history to win the Slam Dunk Contest three times.
Robinson, 40, told Men’s Health that team doctors had warned him of high blood pressure and even went as far as trying to stop him from playing games if his pressure was too high. Diabetes and high blood pressure are the two main causes of kidney disease, according to the National Kidney Foundation.
Knowing he wasn’t going to stop playing, Robinson said he would tell the doctors to stop checking.
In 2006, he was diagnosed with kidney disease. Doctors then warned him that his kidneys would likely fail in his 30s. But Robinson said he “felt like I was Superman.”
“I never thought I would get sick,” he said.
By 2018, he was experiencing kidney failure. Two years later, Robinson said he caught Covid and ended up in the hospital. Doctors told him that his kidneys were working too hard and he needed to start dialysis.
“They told me I might as well start dialysis today,” he said, recalling his 2020 visit. “‘Your kidneys are working too hard; they’re deteriorating as we speak. The only way you will walk out of here alive is if you start dialysis.’ It was the only thing I had left.”
He told the publication that he hopes to get on the Washington state kidney transplant waitlist and eventually the national list.
Robinson said he has accepted his journey, recalling how his mother always knew he was a fighter.
“She knows that I’m gonna give it my all like anything else,” he told the publication. “That’s one thing I respect about myself. I’ve never been a quitter. And I ain’t gon’ start now.”
This story first appeared on NBCNews.com. More from NBC News:
]]>The FDA sent a warning letter to Dollar Tree this month and placed Negasmart, the Ecuadorian distributor of WanaBana apple cinnamon pouches, under import alerts following the October 2023 recall of the products found to be contaminated with “extremely high” levels of lead and chromium.
Children in 44 states had probable or confirmed cases of elevated blood lead levels after eating the applesauce pouches marketed for toddlers, according to the U.S. Centers for Disease Control and Prevention. The outbreak was declared in April.
FDA officials sent a warning letter to Dollar Tree Inc. last week saying the WanaBana apple puree products remained on store shelves in several states through late December, two months after the firm was told about the recall. Officials at the Chesapeake, Virginia-based company had said they disallowed sales of the products at registers, but the FDA said that was “not an effective measure” because at least one child in Washington state ate a recalled fruit pouch in a store before an attempted purchase.
Dollar Tree officials said in an email Tuesday that the company is operating under new management and is taking steps to bolster its process “for quickly and effectively executing product recalls.” The company operates more than 16,000 discount stores in 48 states.
Negasmart was placed under multiple import alerts this month, even though FDA officials said they had no indication that the firm is attempting to import products into the U.S. The action is to ensure that any attempt by the firm to import products would be “flagged” by FDA reviewers and prevented from reaching consumers.
Tests showed cinnamon tested from the plant had lead levels more than 2,000 times higher than the maximum level proposed by the FDA.
Anyone who consumed the recalled pouches should consult with a health care provider, the CDC said. There is no safe level of lead consumption, which can cause serious learning and behavior problems, the agency emphasized.
The pouches include those sold under the WanaBana brand at Dollar Tree stores and online and under the Schnucks and Weis brands in stores. Because they have a long shelf life, they may still be in consumers’ homes. Consumers should not eat or serve the pouches and should discard them.
]]>An analysis of data from more than 500 children and teens with hypertension revealed an association between shorter-than-recommended sleep times and high blood pressure, according to the study, published in Pediatrics.
While hypertension in kids has been declining, the Centers for Disease Control and Prevention estimates that 1 in 7 young people ages 12 to 19 have hypertension.
The study doesn’t prove that shortened sleep times cause hypertension, but doctors don’t typically think of sleep when they counsel parents about high blood pressure, said the study’s lead author, Dr. Amy Kogon, an assistant professor at the University of Pennsylvania Perelman School of Medicine.
Data for the study came from children and teens seen at a clinic at the Children’s Hospital of Philadelphia, where Kogon is a pediatric kidney specialist.
“We usually target things like diet and exercise,” Kogon said. “This is another thing parents might want to think about, particularly if the child has high blood pressure.”
Major risk factors for hypertension in children and teens include being overweight, not getting enough physical activity and a poor diet, according to the American Heart Association.
The majority of middle and high school kids in the U.S. are sleep-deprived. In fact, according to the CDC, nearly 60% of middle school kids and more than 70% of high schoolers aren’t getting enough sleep.
As many as a third of elementary school kids get less than the recommended amount of sleep, Kogon said.
The number of hours of sleep children and teens should get depends on age. The American Academy of Sleep Medicine recommends:
- 10 to 13 hours per night for children under age 6.
- 9 to 12 hours per night for children ages 6-12.
- 8 to 10 hours per night for ages 13 to 18.
- 7 to 9 hours per night for ages 18 and older.
It’s important to control blood pressure early in life because the longer someone has hypertension, the higher the risk of developing heart disease, said Dr. Barry Love, director of the congenital cardiac catheterization program at Mount Sinai Kravis Children’s Heart Center.
“We know that high blood pressure is associated with the early onset of coronary disease and stroke,” said Love, who wasn’t involved in the new study. “We think that the damage to blood vessels happens over time.”
For the new study, researchers at Children’s Hospital of Philadelphia examined the medical records of 539 kids, average age 14.6 years, who were referred to pediatric kidney clinics because of high blood pressure readings. The kids were asked when they went to bed and when they got up in the morning. They were also asked to wear ambulatory blood pressure measuring devices, which took readings every 20 minutes while they were awake and every 30 minutes during sleep.
The further sleep duration was from recommended levels, the more likely it was for kids to experience high blood pressure during the day. Kids who went to bed late were also more likely to have hypertension. The findings were consistent regardless of the kids’ ages, sexes and BMI categories.
Too much sleep was also linked to blood pressure issues. Normally, blood pressure drops by around 10% during sleep, but that was less likely when kids slept longer than the recommended amount.
Why can’t kids sleep?
Anxiety causes sleep problems for about 25% of children ages 1 to 6, according to a University of Michigan C.S. Mott Children’s Hospital National Poll. Those kids were less likely to have bedtime routines and more likely to leave on videos or TV shows, their parents reported in the poll, which was released Monday.
Another possible cause of sleep problems: An estimated 59% of kids weren’t turning off their electronic devices at night, the poll found.
It’s not so much the light from the devices that causes problems but rather what the kids are viewing on such devices. Apps like TikTok and Instagram can spoil sleep because they are “difficult to put down and are also stimulating,” Kogon said.
Reading a book on a device, for example, “is probably not the same as flipping through social media,” Kogon said.
Parents shouldn’t allow cellphones in kids’ bedrooms at night, Kogon said. She also suggests kids not have any kind of electronics or TVs in bedrooms.
Dr. Mariana Bedoya, an assistant professor of allergy, immunology, pulmonology and sleep medicine at Monroe Carell Jr. Children’s Hospital at Vanderbilt University in Nashville, Tennessee, said other ways to help improve sleep include:
- Quitting caffeine at least six hours before bed.
- Keeping to regular sleep schedules.
- Avoiding naps for older kids.
“I tell patients not to change their sleep schedule by more than an hour and a half to two hours over the weekend,” said Bedoya, who wasn’t involved in the new study.
Love said it’s tough for kids to get enough sleep these days. “Is it that kids are worrying or eating bad things?” he asked. “There are so many things distracting them from sleep.”
This story first appeared on NBCNews.com. More from NBC News:
]]>The 45-year-old spoke about his health regimen in an interview with The Wall Street Journal.
Speaking to the outlet, the “Yeah!” singer detailed how his typical routine starts by passing on coffee and reaching for celery juice instead and the ways he prioritizes mental and physical activity. One noteworthy aspect of Usher’s regimen is his weekly fast, something he adopted from his grandmother.
Read on for everything the singer’s said about his wellness habits.
Yoga is a must in the morning
Usher begins each day with intentionality, setting out to wake up early and getting into a reflective and relaxed mindset.
“I try to wake up early enough to have a moment of reflection. Some days I may grab a book and read to stimulate my mind,” he said.
“I may sit quietly and meditate. One thing that is a frequent practice is yoga. It really does help to activate my organs and get my mind moving in the right direction — as Tony Robbins would say, ‘make my move,’ you know what I mean?”
He doesn’t drink coffee
“It all depends on how I ended my night before. Sometimes a coffee martini is appropriate,” he said. “No, typically, I wake up and drink celery juice. I’ve been doing this concoction of lemon, ginger, water and cayenne pepper. I drink it hot.”
He has a go-to breakfast
The singer typically opts for eggs for breakfast, which he usually sits down for after a workout.
“I sometimes eat eggs scrambled with cheese,” he said. “For the most part, I like them poached or over easy. But I don’t like to eat breakfast before I’ve worked out or done something physical: taking a walk, stretching or doing yoga, sitting in the sun and raising my body’s natural heat levels. Then I eat.”
He fasts one day a week
The singer fasts every week. He begins the night before and makes sure he hydrates throughout his fast day.
“I fast, not for religious purposes, but it’s something my grandmother practiced,” he explained. “I fast on Wednesdays. I typically try to start around 11 p.m. the previous day, then go the entire day on Wednesday just drinking water. “
He doesn’t do much weightlifting
Usher explained that his workouts tend to focus on strengthening his knees due to a previous injury.
“Normally, my workout regimen starts either walking or with certain knee activations and reverse walking that I do to really engage my quads, my knees and glutes,” he said.
“I’ve had minor surgeries on my knee, I had a torn meniscus. Other than that, swimming is a really good thing to get me going, and bike riding. Weight lifting, don’t do a lot of that.”
This article first appeared on TODAY.com. Read more from TODAY here:
]]>The Food and Drug Administration on Monday approved Merck‘s new vaccine designed to protect adults from a bacteria known as pneumococcus that can cause serious illnesses and a lung infection called pneumonia, the drugmaker said.
Merck’s shot, called Capvaxive, specifically protects against 21 strains of that bacteria to prevent a severe form of pneumococcal disease that can spread to other parts of the body and lead to pneumonia. It’s the first pneumococcal conjugate vaccine designed specifically for adults and aims to provide broader protection than the available shots on the market, according to the drugmaker.
Healthy adults can suffer from pneumococcal disease. But older patients and those with chronic or immunocompromising health conditions are at increased risk for the illness, especially the more serious or so-called “invasive” form.
Invasive pneumococcal disease can lead to meningitis, an infection that causes inflammation in the area surrounding the brain and spinal cord, and an infection in the bloodstream called bacteremia.
“If you have chronic lung disease, even asthma, you have a higher risk of getting sick with pneumococcal disease, and then being in the hospital, losing out on work,” Heather Platt, Merck’s product development team lead for the newly cleared vaccine, told CNBC in an interview. “Those are things that have a real impact on adults and children, their quality of life.”
Around 150,000 U.S. adults are hospitalized with pneumococcal pneumonia each year, Platt said. Death from the more serious form of the disease is highest among adults 50 and above, Merck said in a release in December.
Even after the FDA approval, the company’s single-dose vaccine won’t reach patients just yet. An advisory panel to the Centers for Disease Control and Prevention will meet on June 27 to discuss who should be eligible for the shot.
Platt said Merck will support the committee’s decision and is ready to supply the vaccine by late summer.
Merck’s competitive edge
Some analysts view Capvaxive as a key growth driver for Merck as it prepares to offset losses from its blockbuster cancer drug Keytruda, which will lose exclusivity in the U.S. in 2028.
The market for pneumococcal conjugate vaccines is currently around $7 billion and could grow to be worth more than $10 billion over the next several years, according to a November note from Cantor Fitzgerald analysts.
Merck’s newly approved shot could boost its competitive edge in that space, which includes drugmaker Pfizer. Merck currently markets two pneumococcal shots, but neither is specifically designed for adults. For example, the company’s existing shot Vaxneuvance is approved in the U.S. for patients 6 weeks of age and older.
Pfizer’s single-dose pneumococcal vaccine, Prevnar 20, is the current leader in the market for adults. But Merck expects its new shot to capture the majority of market share among adults, Platt said.
“We do expect there to be rapid uptake of” Capvaxive, she said, adding that the company is confident that data on the shot will “really resonate” with clinicians and policymakers.
Merck’s pneumococcal vaccine protects against eight strains of the bacteria that are not included in any other approved shot for the disease. Those eight strains account for roughly 30% of invasive pneumococcal disease cases in patients 65 and above, according to a release from Merck, citing CDC data from 2018 to 2021.
The 21 strains included in Merck’s shot account for roughly 85% of invasive pneumococcal disease cases in adults 65 and above, Merck, citing the CDC data. Meanwhile, Pfizer’s Prevnar targets strains that only account for roughly 51% of cases in that age group, based on the same CDC data.
The FDA’s approval is partly based on Merck’s late-stage trial called STRIDE-3 that pitted the vaccine against Pfizer’s Prevnar 20 in adults 18 and up who had not previously received a pneumococcal vaccine.
Correction: This story has been updated to reflect 150,000 U.S. adults are hospitalized with pneumococcal pneumonia each year.
]]>The FLiRT strains — which include KP.3, KP.2, and KP.1.1 — now account for more than half of all COVID-19 infections nationwide, according to the latest data from the U.S. Centers for Disease Control and Prevention.
These new variants, which scientists dubbed “FLiRT” after the locations of their spike protein mutations, have been circulating in the U.S. since the early spring. In April, KP.2 quickly overtook JN.1, the omicron subvariant that drove a surge in COVID cases this past winter.
In a matter of weeks, the KP.3 variant overtook KP.2 to become the most prevalent strain in the U.S., per the CDC.
During a two-week period ending on June 8, KP.3 made up an estimated 25% of cases in the U.S., up from about 9% in early May. After KP.3, the next most common variant is KP.2, another FLiRT variant that gained dominance in May and now accounts for about 22% of cases. It’s followed by LB.1, a JN.1 subvariant, and another FLiRT variant, KP.1.1.
Together, the FLiRT variants make up an estimated 55% of cases in the U.S.
Although hospitalizations are down and COVID-19 numbers are relatively low, there has been a small uptick in test positivity and emergency room visits, per the latest CDC data. These trends, along with previous summer waves, have stoked fears about a surge of infections this summer.
Scientists are warning that the FLiRT variants may be better at evading the immune system due to their spike protein mutations, and that waning immunity and poor uptake of the latest COVID-19 vaccine have created a more susceptible population.
Will there be another COVID-19 wave? What are the symptoms of the FLiRT variants? Are vaccines still effective? We spoke to experts to learn more.
What is KP.3?
KP.3 is one of the FLiRT variants — along with KP.2 and KP.1.1 — which are spinoffs of JN.1.11.1, a direct descendant of JN.1. They were initially detected in wastewater samples from across the country.
KP.3 and the other new variants have additional mutations that set them apart from JN.1 and appear to give them an advantage over previous variants, Dr. Albert Ko, infectious disease physician and professor of public health, epidemiology and medicine at Yale School of Public Health, tells TODAY.com.
The nickname “FLiRT” is based on the technical names for their mutations, according to the Infectious Disease Society of America.
Just like other COVID-19 strains that have gained dominance in the U.S. over the last year — JN.1, HV.1, EG.5 aka Eris, and XBB.1.16 or Arcturus — the FLiRT variants part of the omicron family.
The emergence of KP.3 and other FLiRT variants is the “same old story,” Andrew Pekosz, Ph.D., virologist at Johns Hopkins University, tells TODAY.com. The SARS-CoV-2 virus mutates and gives rise to a new, highly contagious variant, which becomes the dominant strain. “The timeline that it happens in, three to six months, is much faster than we see with other viruses like influenza,” says Pekosz.
Is KP.3 more transmissible?
“It’s still early days, but the initial impression is that this variant is rather transmissible,” Dr. William Schaffner, professor of infectious diseases at Vanderbilt University Medical Center, tells TODAY.com.
The proportion of cases caused by KP.3 is increasing, while the proportion caused by other variants is decreasing, which suggests the FLiRT variants have features that give it an advantage, the experts note.
Over 97% of people in the U.S. have natural or vaccine-induced antibodies against the the SARS-CoV-2 virus, per the CDC, but this immune protection fades over time.
Low vaccination rates and waning immunity create a vulnerable population, which may allow the FLiRT variants to take hold. Only time and more data will tell, the experts note.
Laboratory studies suggest that the FLiRT variants are mutated enough such that current vaccines and immunity from prior infection will only provide partial protection, says Schaffner. “We’ll have to see how true that is, but it appears, over time to be becoming a more prominent variant,” he adds.
“It’s still really early … but I don’t think we need to sound the alarm bells as of yet,” says Ko.
The state of COVID in the U.S.
It’s too soon to tell whether KP.3 and the FLiRT variants will cause a summer wave or surge, the experts note. However, it is clear that COVID-19 is still circulating and won’t be taking any time off.
“We’re seeing these infections year-round, at modulating levels. … We’re probably not at the stage yet where we’ll see COVID go away completely at any time of the year,” says Pekosz.
Test positivity, which is an early indicator of case levels, was 5.4% as of June 8, up 0.8% from the previous week but a sharp decline from a peak of about 12% in mid-January, per the CDC. (The CDC no longer tracks the total number of cases in the U.S.)
“We’re not seeing a lot of hospitalizations, and we’re certainly much lower than we were in the winter, so I’d say right now we’re at a low point, which is reassuring,” says Ko.
Wastewater data published by the CDC show that the viral activity level for COVID-19 is currently “low” — it was considered high or very high for most of January and February.
“It seems like transmission is pretty low right now, and that makes sense because usually the big peaks are in the winter, when people are inside and in more contact,” says Ko.
COVID-19 has caused summer waves in the past, the experts note, which are often smaller than the winter surges. “I don’t think that we’ll see any kind of massive surge in cases,” says Pekosz.
The seasonality of COVID-19 is something scientists are still trying to understand. But one thing is obvious: “This virus is now integrating itself into our population and our way of life,” says Schaffner.
Adds Ko: “I’m not expecting a large surge in the summer, but again, we have to be cautious and we have to follow the data. … We always have to be humble because SARS-CoV-2 has taught us a lot of new things.”
What are the symptoms of KP.3?
It is still too early to tell whether the symptoms of KP.3, KP.2 and other FLiRT variants are different from previous strains.
“The FLiRT variants are probably not going to create very distinctive symptoms. It looks at the moment to follow the other subvariants,” says Schaffner.
The symptoms of the FLiRT variants are similar to those caused by JN.1, which include:
- Sore throat
- Cough
- Fatigue
- Congestion
- Runny nose
- Headache
- Muscle aches
- Fever or chills
- New loss of sense of taste or smell
- Shortness of breath or difficulty breathing
- Nausea or vomiting
- Diarrhea
According to the CDC, the type and severity of symptoms a person experiences usually depend more on a person’s underlying health and immunity rather than the variant that caused the infection.
Similar to JN.1 and other omicron subvariants, the FLiRT variants seem to be causing milder infections, says Schaffer.
Do vaccines protect against KP.3?
Early laboratory studies indicate that the vaccines will continue to provide protection the FLiRT variants — “a little less protection, but not zero by any means,” says Schaffner.
As the virus mutates, it is becoming progressively different from the omicron strain targeted in the latest updated booster released in the fall of 2023. “We would expect that to happen, and we anticipate the plan is to have an updated vaccine in the fall available to everyone,” says Schaffner.
Advisers to the U.S. Food and Drug Administration met on Tuesday, June 5, to decide which strains to include in the updated COVID-19 vaccines for 2024–2025. The committee unanimously voted to recommend a monovalent vaccine targeting the JN.1 variant for this fall, the agency said in a press release.
Even if vaccines do not prevent infection, they can still offer some protection by preventing severe disease, hospitalization, and COVID-19 complications, TODAY.com previously reported.
“It’s still clear that the more severe cases that come into the emergency room predominate in people who either are not up to date on their vaccines or haven’t gotten a vaccine in a really long period of time,” says Pekosz.
Vaccination is especially important for the elderly, says Pekosz, which is why the CDC recently recommended adults ages 65 and older get an additional dose of the 2023-2024 updated COVID-19 vaccine.
Unfortunately, vaccination uptake is still poor, the experts note. “The vaccines are still showing signatures of effectiveness, but they’re not being utilized anywhere close to the level that they should be,” says Pekosz.
As of April 2024, only about 22% of adults and 14% of children have reported receiving the updated COVID-19 vaccine released in September 2023, according to the CDC.
All current PCR and at-home tests are recognizing KP.3 and other FLiRT variants, the experts note. (Though if you have symptoms of COVID and test negative, it’s a good idea to stay home to avoid potentially exposing other people, TODAY.com previously reported.
If you are using an at-home antigen test, always remember to check the expiration date and whether it’s been extended by the FDA.
“Antivirals (such as Paxlovid) are also working well. … There’s not any major signals of antiviral resistance in the population, which is a positive sign,” says Pekosz.
How to protect against KP.3 and FLiRT variants
While it’s too early to tell how the FLiRT variants will pan out this summer, people can always take steps to protect themselves and others against COVID-19.
The CDC recommends the following prevention strategies:
- Stay up to date with COVID-19 vaccines.
- Test for COVID-19 if you have symptoms or an exposure.
- Stay home when you are sick.
- Return to normal activities only after you have been fever-free and symptoms have been improving for at least 24 hours.
- Practice good hand hygiene.
- Improve ventilation.
- Wear a mask in crowded, indoor spaces.
- Practice social distancing.
This story first appeared on TODAY.com. More from Today:
]]>A study looked at how dust and debris from the aftermath of the World Trade Center attacks has affected the brain health of first responders.
Researchers from Stony Brook University found exposure to toxins at Ground Zero was associated with higher risk of dementia before the age of 65. That risk was much lower for those who were not exposed or who wore protective equipment.
The study, which was comprised of more than 5,000 responders, adds to previous research showing 9/11 first responders show signs of cognitive impairment at about three times the rate of the general population.
Among those who participated, there were 228 responders under the age of 60 without dementia at the start of the study who developed the illness over the course of the next five years. That equates to 4.6% of responders in the study; lead author Sean Clouston said that in the general population, the incidence would only be about 0.5%.
Additionally, in responders who reported no dust exposure or those who wore protective equipment like masks and hazmat suits, about five or six out of every 1,000 in the study developed dementia each year. But for the responders who did not wear PPE and had reported doing things like digging through the World Trade Center debris, the dementia rates topped 42 per 1,000 people.
“This rate of dementia in those reporting many exposures and limited protection is not only statistically significant, it is alarming for a patient cohort that clearly shows a strong association between exposure and the incidence of dementia under the age of 65,” says Clouston. “Also, the rates remained statistically significant over the less exposed group even after adjusting for social, medical, and demographic factors.”
]]>The CDC said four people have been hospitalized in the multi-state outbreak. The majority of people who faced the illness were children under five years old, the CDC said.
Symptoms of salmonella can include diarrhea, fever, and stomach cramps.
The CDC said people handing bearded dragons or their habitats should always wash their hands thoroughly with soap and water, and warned against kissing or snuggling a bearded dragon. Health officials said to avoid eating and drinking around the reptiles too and keeping them away from spaces where young children eat or play.

Bearded dragons are not recommended for families with children younger than five-years-old, people 65 years of age and older, or people with a weakened immune system, according to the CDC, which said people in those categories are more susceptible to illnesses from reptiles.
Four people have been sickened in New York. Other states impacted are: California, Georgia, Iowa, North Carolina. Ohio, Oklahoma, Tennessee and Texas.
]]>High up in the hills of Barcelona, brain science start-up Neuroelectrics is developing therapies that it says will improve the lives of people living with brain disease.
The group manufactures around 400 devices, which it ships to 75 different countries worldwide each year.
Its main product is a headcap, which monitors the brain’s electrical activity and stimulates regions of the brain with mild electrical currents.
Co-founder and CEO Ana Maiques believes it will significantly improve the lives of people suffering with epilepsy.
“In the world, there are 60 million patients suffering from epilepsy and one third of those don’t respond to medication,” Maiques told CNBC Tech: The Edge in an interview.
“These patients usually go into surgery, either a craniotomy — we remove the part of the brain that is creating the seizures — or an implanted device. So Neuroelectrics is bringing this noninvasive solution to try to reduce seizures.”
In a 17-patient study approved by U.S. Food and Drug Administration, the technology demonstrated a median seizure reduction of 41%. Neuroelectrics is pursuing FDA approval by September 2025.
In addition to epilepsy, Maiques and her team are optimistic that the headgear can also be used to treat depression and Alzheimer’s.
“Our devices read the electrical activity of the brain, but also inject electricity. So, the areas that we’re focused on are those that are clearly electric. So, when you have epilepsy, you have an electrical discharge in one area of your brain, so they can really target and help them,” said Roser Sanchez-Todo, R&D director for Neuroelectrics’ brain modelling department.
NeuroTwin
Before a patient can use the technology, Neuroelectrics builds a replica of their brain, known as a NeuroTwin.
“We’ve been using what is now called AI, or machine learning, for years. If you think of flight pilots, they don’t go into planes, they are in simulators. So, why can you not have a simulator of the brain, where you can really have a digital copy of your brain?” Maiques said.
“Then we can say, if we provide you this treatment, or this stimulation, how is your brain going to react? We are very excited about our NeuroTwin technology. I think it’s going to change the way we look at brain diseases,” she added.
The end goal is for patients to be able to use the cap at home, which is essential as it takes ten daily 20-minute sessions for around eight weeks for the average epilepsy patient to feel results.
“Then you just go. You put your headcap on, maybe you need some help in order to put the gel for the electrodes, and you just press the start stimulation,” Sanchez-Todo said.
“Usually, it’s from 20 minutes to an hour that you’re sitting and relaxing. Then you just need to take it out, clean it, and then for the next day you repeat.”
]]>Ruthia He, the founder and CEO of Done Global Inc., allegedly conspired with the company’s clinical president, David Brody, and others to provide easy access to stimulants, including Adderall, a drug used to treat ADHD, in exchange for payment of a monthly subscription fee, the Justice Department said in a news release.
He was arrested in Los Angeles and Brody in San Rafael, California on charges of conspiracy to distribute controlled substances and distribution of controlled substances. If convicted, they each face a maximum sentence of 20 years in prison. It’s not clear if He and Brody have obtained attorneys who can speak on their behalf.
He and Brody ran the scheme to “unlawfully enrich themselves” and made over $100 million by increasing monthly subscription revenue, which therefore increased the value of the company, federal officials said.
Principal Deputy Assistant Attorney General Nicole M. Argentieri accused He and Brody of exploiting telemedicine “and spending millions on deceptive advertisements on social media.”
“They generated over $100 million in revenue by arranging for the prescription of over 40 million pills,” Argentieri said in a statement. “These charges are the Justice Department’s first criminal drug distribution prosecutions related to telemedicine prescribing through a digital health company. As these charges make clear, corporate executives who put profit over the health and safety of patients — including by using technological innovation — will be held to account.”
The pair allegedly obtained subscribers by spending millions on what officials called deceptive social media advertisements, targeting drug seekers, and intentionally structuring the Done platform to facilitate access to Adderall and other stimulants, the news release alleges.
Part of the scheme allegedly included limiting the information available to Done prescribers and instructing them to prescribe Adderall and other stimulants even if the Done member did not qualify.
He tried to maximize profits by adding an “auto-refill” function that allowed subscribers to elect to have a message requesting a refill of the drug every month, the Justice Department said.
He and Brody are also accused of conspiring to defraud pharmacies as well as Medicare and Medicaid.
The Justice Department said that He and Brody allegedly continued with the scheme even after being made aware that Done members had overdosed and died and that there was material posted online about how people could obtain Adderall and other stimulants from the company.
Done, which says it makes high-quality psychiatric chronic care more affordable and accessible, did not immediately respond to a request for comment on Thursday. He and Brody could not be reached at phone numbers listed for them.
In 2022, the Wall Street Journal reported that some clinicians said they felt that the company was pressuring them into prescribing stimulants. That same year, the Drug Enforcement Administration opened an investigation looking at Done’s practice of prescribing controlled substances, the WSJ reported.
The DEA lists Adderall as a schedule II drug with a high potential for abuse. It is included in the same category as Vicodin, OxyContin, and methamphetamine.
This story first appeared on NBCNews.com. More from NBC News:
]]>In an alert Wednesday to health care providers, the CDC said that a dozen people in eight states have gotten sick after eating the brand’s “microdosing” mushroom edibles. All but two needed to be hospitalized.
Symptoms included seizures, sedation, muscle stiffness, abdominal pain, abnormal heart rate and high or low blood pressure. None died, but several patients needed to be put on ventilators and were admitted to the intensive care unit, the CDC said.
The actual scale of the outbreak may be even larger, according to Kait Brown, clinical managing director of America’s Poison Centers. Nationwide, poison centers have received 22 reports of illnesses potentially linked to Diamond Shruumz products and are seeing new cases every day, Brown said.
The CDC tally represents the most severe cases, she said, but poison centers are also aware of milder cases that were limited to gastrointestinal symptoms like nausea or vomiting, or feelings of sleepiness or light sedation.
Why are Diamond Shruumz potentially making people sick?
Diamond Shruumz says on its website that its products are meant for microdosing. Microdosing typically refers to consuming small amounts of psychoactive or hallucinogenic substances, enough to reap the benefits while minimizing more debilitating effects.
However, the company also says on its website that its products don’t contain psychedelic substances. Several toxicology experts said the mushrooms listed as ingredients, such as lion’s mane or ashwagandha, don’t produce the potent effects that the company touts, like relaxation or euphoria.
“The mushrooms that they advertise that are in them are pretty innocuous and in a lot of other products that don’t make similar claims,” said Maryann Amirshahi, co-medical director of the National Capital Poison Center in Washington, D.C.
The CDC said in its alert that products containing psychoactive ingredients, such as mushroom extracts, are becoming more available and are often sold as gummy candies, chocolate or other snack foods. Such products may contain “undisclosed ingredients, including illicit substances,” the CDC said, “or potentially harmful contaminants that are not approved for use in food.”
Diamond Shruumz did not respond to a request for comment.
“At this point, what is causing these symptoms in these products are still under investigation,” Brown said. “It does speak to the uncertainty about what is in the product that could make such a wide array of symptoms.”
Are mushroom chocolates regulated?
The market for mushroom-based products such as coffee or chocolate has swelled in recent years, as has demand for edibles with psychoactive properties, though psychedelic mushrooms are largely illegal in the U.S..
Toxicology experts said Diamond Shruumz products likely fall under the category of a dietary supplement based on their listed ingredients and how they’re advertised.
Dietary supplements don’t require approval from the Food and Drug Administration before being sold to customers, though the agency mandates that companies that manufacture, package, label or store supplements test their ingredients and limit contamination.
“It’s not like a prescription medication where you have to prove safety and efficacy before you can sell it,” said Steven Dudley, director of the Arizona Poison and Drug Information Center.
“With supplements, really it’s the other way around where there has to be reports of harm for the powers that be to be able to step in and then issue recalls, take products off the market, et cetera.”
That lack of regulatory oversight means that quality control is “murky at best,” said Dr. Chris Hoyte, medical director of the Rocky Mountain Poison Center in Denver.
In the past, some dietary supplements have been found to contain undisclosed ingredients that may cause harm. For instance, the FDA warned last year that yellow oleander — a poisonous plant — was masquerading as supplements sold for purported weight loss benefits.
Amirshahi said the makeup of dietary supplements can also vary from batch to batch, which in theory could explain why Diamond Shruumz customers had a range of symptoms.
“At low doses, you may see one thing and higher doses may see the other,” Amirshahi said. “So at lower doses, they may be kind of really agitated, off the rails, have high blood pressure, have a high heart rate — but if they start taking more and more of it, the effects actually change.”
Experts also said it’s possible that psychedelics were illegally added to the products, or that the chocolates and gummies contained other legal substances that weren’t disclosed.
“We don’t know if this is a bad batch issue,” Dudley said. “We don’t know if it’s more widespread than that. What we do know is that it’s causing harm, so we’d really urge the public to not use these products.”
The FDA said Tuesday that consumers should discard and refrain from eating any flavor of Diamond Shruumz chocolate bars, cones or gummies. In a statement, the agency added that it “will continue to monitor the marketplace to identify products that pose risks and will take action within our authorities against unlawful products in order to protect the public.”
This story first appeared on NBCNews.com. More from NBC News:
]]>Despite the ruling, women’s access to mifepristone still largely depends on a patchwork of state laws, with only about half of states allowing full access under terms approved by the federal government.
“It doesn’t change anything anywhere,” said David S. Cohen, a law professor at Drexel University. “Tomorrow’s the same as today, which is the same as yesterday, which is the same as before this case was filed.”
Here’s a look at what Thursday’s decision does and does not mean for abortion access.
What did the Supreme Court decide?
Essentially, the justices said the anti-abortion doctors who brought the case did not have the legal standing to sue the Food and Drug Administration over the drug’s safety or changes making it more widely available. The FDA approved the drug more than 20 years ago and has reiterated its safety and effectiveness.
The anti-abortion doctors, under the name the Alliance for Hippocratic Medicine, argued they might have to treat emergency room patients who experience serious injuries after taking mifepristone.
While the decision keeps mifepristone available, legal experts say that other groups or individuals who believe they can show a stronger legal connection to the drug might try to sue along similar lines.
“It’s a win that the status quo is preserved but it doesn’t signal that these are now dead arguments that others aren’t going to try and pursue,” said Rachel Rebouche, a Temple University law professor.
What is mifepristone?
Mifepristone is prescribed to end pregnancies by dilating the cervix and blocking the hormone progesterone, which is needed to sustain a pregnancy. It is usually taken with a second drug, misprostol, that causes the uterus to cramp and contract. The two-drug regimen is used to end a pregnancy through 10 weeks.
What does the ruling mean for the status of mifepristone?
Mifepristone remains fully approved and available under the current FDA framework, which allows telehealth prescribing and mail delivery to patients. The FDA has also expanded availability to large pharmacy chains and allowed prescribing by nurses and other health professionals.
Those policies have increased the prescribing of mifepristone, which accounted for nearly two-thirds of all U.S. abortions last year.
How do state laws impact access to mifepristone?
Access to the pills is restricted across large swaths of the country because of state laws that ban abortion (including medication abortion) outright or impose separate restrictions on the drug’s use.
Access largely depends on the laws in the state where a patient lives and, in the case of states banning or restricting mifepristone, what steps they are willing to take to circumvent them.
About half of U.S. states allows online prescribing and mail delivery of mifepristone, conforming to FDA’s drug label.
Currently, 14 states are enforcing bans on abortion at all stages of pregnancy, including with mifepristone. More than a dozen other states have laws specifically limiting how it can be prescribed, such as requiring an in-person visit with a physician or separate counseling about the potential risks and downsides of the drug.
Those steps are not supported by major medical societies, including the American Medical Association.
How safe and effective is mifepristone?
The FDA and the Biden administration filed multiple legal challenges reiterating the drug’s safety and effectiveness.
Mifepristone results in a completed abortion 97.4% of the time, according to the FDA label. Like all drugs, the abortion pill is not 100% effective and in 2.6% of cases, a surgical intervention was needed to complete the abortion. Less than 1% of the time, the pregnancy continued.
In rare cases, mifepristone can cause serious complications including excessive bleeding, infections and other emergency problems. Those occur in far less than a fraction of 1% of all patients using the drug, according to the FDA label.
How are medication abortions increasing despite restrictions?
Despite state laws targeting mifepristone, statistics show women in those states continue to receive the drug through the mail because state authorities have little visibility into deliveries by the U.S. Postal Service.
A survey earlier this year found about about 8,000 women a month in states that restrict abortion or place limits on prescribing were getting the pills by mail at the end of 2023, according to the Society of Family Planning.
What’s next for legal challenges to mifepristone?
Legal experts say other parties could bring new lawsuits.
Idaho, Kansas and Missouri sought to join the case against the FDA, which the Supreme Court rejected — though a conservative Texas judge who initially ruled against the FDA allowed them to intervene in his district. The three states, all led by Republican attorneys general, could try to revive the case at the lower court, according to legal experts.
“They are not physicians who have to show that they actually have some relationship to abortion care,” Rebouche said. “They’re claiming a state interest in the regulation of medicine, so I think that’s the vehicle in which you could see a lawsuit come forward.”
___
Geoff Mulvihill contributed to this story from Cherry Hill, New Jersey
]]>“It started with the numbness in my feet, almost like my shoes were too tight, and it progressed to where I was having trouble walking,” Dugal, now 34, of Winston-Salem, North Carolina, tells TODAY.com. “I knew there was something significantly wrong.
Dugal, who had just finished his surgery residency, went to a local hospital, where he learned he had a rare post-viral complication called Guillain-Barre syndrome. It can cause anything from muscle weakness to complete paralysis, and very few interventions can slow its progression.

“I couldn’t even move my eyes and blink. And as that’s happening, I can’t express enough the fear and uncertainty I had,” Dugal says. “Sometimes medical knowledge is a good thing and a bad thing because you are keenly aware of the severity of your illness.”
COVID-19 infection leads to numbness and ‘strange’ symptoms
Over Labor Day weekend 2022, Dugal and his family had a lot to celebrate. He had just completed his four-year surgical residency and was preparing to start a new job in North Carolina. His wife also recently had given birth to a beautiful baby daughter.
“Things were really looking great,” he says. “(We) were about to start the next chapter.”
They attended a wedding, and after returning home, all three tested positive for COVID-19. Dugal’s wife and daughter had mild cases, but his symptoms were “strange,” such as foot numbness, Dugal recalls.
Over the next several days, the numbness worsened, so he asked his wife to take him to the hospital. “I had to be wheeled in because I couldn’t walk at all,” he says.
A neurologist ordered a spinal tap, which helped doctors quickly diagnosis Dugal with Guillain-Barre syndrome, a rare condition where the immune system attacks the layer around the nerves (myelin), causing nerve damage, according to the National Institute of Neurological Disorders and Stroke.
“Unfortunately, my symptoms progressed over a period of month in the hospital with complication upon complication,” he says.
How Guillain-Barre syndrome progresses
In mild cases, Guillain-Barre syndrome only causes muscle weakness. In more severe ones, it progresses to full paralysis, and patients require ventilation to breathe. The amount of time the condition lasts can vary, too, Dugal says.
Most people recover completely or only have minor symptoms, such as numbness or tingling, afterward, according to Mayo Clinic. But recovery can take months to years. For people who lose the ability to walk, it usually returns within six months.
The condition can also be fatal, especially if the paralysis moves into the muscles used to breathe. And “sometimes the nerves … are damaged to a point where they’re unable to recover,” Dugal explains. In these cases, patients stay paralyzed.
The worse the early symptoms, the greater the likelihood of long-term complications, per Mayo Clinic.
Experts remain unsure why some people develop Guillain-Barre syndrome, but it most often occurs after bacterial or viral infections. There’s no cure or definitive treatment, so doctors usually offer supportive measures, such as ventilation and feeding tubes, Dugal explains.
“You don’t know how severe it’s going to get, and you don’t know how long it’s going to last,” Dugal recalls of his experience. “They were two kinds of anxiety for me.”
Ventilation leads to a near-death experience
After his receiving diagnosis in the hospital, Dugal felt “keenly aware” of how serous his Guillain-Barre syndrome was.
“I knew that once it progressed high enough to my diaphragm that I wasn’t going to be able to breath,” he says. “It was a very humbling feeling when you realize you’re at the mercy of the process and you have to accept whatever comes.”
He gradually experienced so much weakness his muscles that he could no longer speak. He remembers trying to concentrate his muscles on being able to breathe on his own, but “after a few days, I wasn’t successful,” Dugal says.
Doctors placed him on a ventilator to assist his breathing. At the time, Dugal worried that he would never recover.

“I made peace that I was likely going to die,” he says. “I looked at (my wife) and told her to take care of our daughter.”
There were moments, though, where Dugal’s medical training took over. After he lost his ability to speak, he blinked to communicate, and a few times he tried managing his own treatment.
“I was trying to spell out different ventilator modes,” he says, with a laugh. “I was actively involved in my care.”
After two weeks on the ventilator, Dugal developed pneumonia — a common side effect of being on a ventilator for a prolonged period — and both of his lungs collapsed. His oxygen levels became dangerously low, and he wasn’t getting enough oxygen to his brain, which can be fatal if not addressed quickly.
He began to code, and doctors put him under and placed him on ECMO, a machine that takes over heart and lung function to give them time to recover. After nine days, he awoke.
“I (was) completely cognitively there in understanding,” he recalls. “I (had) these large plastic tubes with all my blood running through them, and I (was) completely dependent on this system working. You can imagine my anxiety was through the roof.”
The ECMO had allowed his lungs to heal, though, so he was weaned off and placed back on a ventilator. Still, he couldn’t speak, wiggle his fingers or toes, or even blink. But he knew exactly what was happening.
“Your muscles are so weak,” he says. “I was completely trapped in my own body and sitting there, staring at the same spot on the wall.”
Dugal began wondering what life would be like. Would he ever be strong enough to return to work as a surgeon?
Because his condition was no longer getting worse, doctors recommended in-patient rehabilitation, but Dugal’s family struggled to find a facility that would take him while he was still on a ventilator. Finally, TIRR Memorial Hermann in Houston accepted him, so he took an air ambulance. Once there, he began working to relearn everything.
Two months in in-patient rehab
Rehabilitation felt difficult. He had lost 60 pounds and was still being fed through a feeding tube because he was too weak to swallow. He couldn’t sit up alone or leave the bed, so they used lifts to transport him. Good days often included incremental changes so slight they could be hard to see.
“It was little things that would be like trying to straighten your hands out … because your muscles literally aren’t strong enough to open,” he says. “I remember the first time I could kind of wiggle my big toe. … It was the most unexciting thing you’ve ever seen.”
Despite the challenges, in rehab Dugal felt like he could “take control of the situation” for the first time since becoming sick. “(At first), you’re in survival mode and trying to get to the next hour,” he says. “(Rehabilitation) was very slow, but there was progress.”
After two months of in-patient rehabilitation, Dugal went home. He was using a power wheelchair and still needed loads of in-home physical, occupational and speech therapy to relearn daily tasks.
“I was trying to get back my life skills,” Dugal says. “To be able to get dressed, to eat by myself … tie (my) shoes, pick up objects.”
Over time, he built up his strength to the point where returning to work felt possible. Nine months after being diagnosed with Guillain-Barre syndrome, he could walk again.

Working as doctor again meant he needed to practice his surgery skills. His wife found a company, Osso VR, that had surgical training programs using VR headsets.
“You could kind of perform surgeries that look like we’re in the operating room and go through the steps of the operation,” Dugal explains. “It was a way to bridge the gap of having physical limitations but also trying to get back to that (surgeon) mindset.”
From patient to doctor
In July 2023, almost a year after he caught COVID-19, Dugal felt strong enough to work. He started working in a lab where surgical studies were being conducted, “trying to figure out how to get back to being a surgeon,” Dugal says.
Then he started an ECMO fellowship, where, for almost a year, he was “putting patients on the same treatment that saved me at the same hospital.” It felt like a full circle moment.
“It was great to be able to work with the same people who saved me — therapists and surgeons,” he says. “I’m very grateful to be able to do surgery.”
When Dugal finishes his ECMO fellowship, he’s going to start a general surgery fellowship.

Having Guillain-Barre syndrome changed his perspective as a doctor.
“I have more empathy and a better understanding of the patient’s experience,” he says. “I hope that I can provide that same compassion and support to other people in similar situations.”
Being able to care for others who need ECMO after it saved him has felt like an honor for Dugal.
“It’s been very rewarding to do ECMO,” he says. “What I want to carry forward in my practice is having frank conversations but also exploring all options in providing hope.”
This article first appeared on TODAY.com. Read more from TODAY here:
]]>Choose the right type of chocolate and you also get a rare dessert that gets approval from dietitians.
June is National Candy Month, though chocolate really rules in October for Halloween, December for the holiday season, February for Valentine’s Day and spring for Easter.
But people love it year-round: the average American eats almost 10 pounds of chocolate per year, according to Forbes.
Many might not know chocolate comes from a fruit tree and is made from a seed — the cocoa bean, the National Confectioners Association notes.
What is the healthiest chocolate?
Of the three types of chocolate — dark, milk and white — dark chocolate is the healthiest, nutrition experts say.
“The health benefits of chocolate products are all thanks to the cocoa bean, which contains numerous phytochemicals shown to have anti-inflammatory, anticancer, and antihypertensive properties,” Whitney English, a registered dietitian at Whitney E. RD in Palo Alto, California, tells TODAY.com.
“The more cocoa solids a product contains, the more nutritious it is. Dark chocolate contains the most cocoa bean solids and therefore is the most nutrient-dense.”
Dark chocolate also has a higher content of flavonoids than milk or white chocolate, says Elisabetta Politi, a registered dietitian at the Duke Lifestyle and Weight Management Center in Durham, North Carolina.
Flavonoids function as antioxidants to block the damaging effects of free radicals, which have been linked to increased risk of heart disease and cancer, she notes.
“Additionally, flavonols, a type of flavonoids in dark chocolate, may affect the function of the immune system by reducing inflammation,” Politi tells TODAY.com.
Is 70% dark chocolate healthy?
Both experts recommend choosing chocolate with at least 70% cocoa content because it will have less added sugar and more phytochemicals than chocolate with less cocoa.
A 70% chocolate bar will list cocoa beans or one of its derivatives — cocoa solids or cocoa liquor — as the first ingredient, Politi says. If sugar is listed first, it means cocoa makes up less than 50% of the bar, she adds.
Dark chocolate benefits
Cocoa beans contain protein and are a great source of minerals like iron and magnesium, plus manganese, copper, zinc and phosphorus, TODAY.com previously reported. You get a bit of fiber, too — about 3 grams per 1 ounce of dark chocolate, according to the U.S. Department of Agriculture.
Chocolate is rich in polyphenols, beneficial compounds produced by plants.
Higher chocolate intake is associated with a lower risk of future heart problems, researchers reported in the journal Heart.
Reviews of studies have found chocolate consumption “significantly reduced” triglycerides — a type of fat in the blood — and can modestly lower blood pressure.
Cocoa flavanols protect against vascular disease and appear to improve blood flow to the brain, a study published in Scientific Reports noted.
Chocolate also has benefits for the mind.
Dark chocolate “contributes to producing the feel-good hormone serotonin and contains magnesium, which is linked to reducing anxiety” and relieving stress, Keri Glassman, a registered dietitian in New York, notes.
Eating 85% cocoa dark chocolate may also boost mood via the gut-brain connection, with dark chocolate having a prebiotic effect on healthy bacteria in the gut and possibly improving negative emotions that way, a study found.
Could it make you smarter? There’s a “surprisingly powerful” correlation between chocolate intake and the number of Nobel laureates in various countries — perhaps because chocolate “enhances cognitive function,” a study published in The New England Journal of Medicine found.
For example, Switzerland was the top performer when it came to both the number of Nobel laureates and the amount of chocolate its residents eat, the authors noted. (Other experts were very skeptical of the correlation.)
Dark chocolate side effects
When Consumer Reports tested 28 dark chocolate bars from a variety of brands in 2022, it found cadmium and lead in all of them — two heavy metals harmful to health. The levels weren’t extremely high, but they were detectable, the organization said when it released its test results.
The National Confectioners Association countered that chocolate is safe to eat and all the products tested were “in compliance with strict quality and safety requirements.”
Any harms from heavy metals seem to be outweighed by other positive compounds in dark chocolate, English notes.
If heavy metals are a concern, Politi suggests choosing milk chocolate, or varying both milk and dark.
Dark chocolate contains caffeine — about 23 milligrams in a 1-ounce square, according to the U.S. Department of Agriculture. If you eat four squares, that’s about the same amount of caffeine as drinking a cup of coffee.
And it’s still candy — it has fat and sugar, with 170 calories per ounce, so eating too much can lead to weight gain.
How much chocolate per day is OK to eat?
Politi recommends sticking to 1 ounce per day, or the size of a dental floss case.
English says a few squares of chocolate a day is a reasonable amount for most people.
How do you eat dark chocolate if you don’t like it?
If it’s too bitter, try putting two small pieces in your mouth and let them melt over your tongue, which helps discover the complexity of the dark chocolate flavor, Politi advises.
A dark chocolate bar that contains sea salt or dried fruit may also taste less bitter than plain dark chocolate, even if they contain the same amount of cocoa, she adds. Politi personally loves chocolate with orange flavor added.
Yogurt with fresh berries and some dark chocolate chips sprinkled on top is another option, English notes.
Both dietitians are fans of dipping fruit in melted chocolate.
Is chocolate unhealthy or healthy?
Dark chocolate contains nutritious components and its benefits likely outweigh any potential drawbacks as long as it’s consumed in moderation, English says.
If a person enjoys a sweet treat at night, choosing a few squares of dark chocolate over a bowl of ice cream is more beneficial, but it’s likely less healthful than a bowl of blueberries, she explains.
“If someone loves a treat at the end of a meal, I think a small amount of dark chocolate is a guiltless choice, which has been shown to provide health benefits,” Politi adds.
“(But) I wouldn’t say chocolate is a health food.”
This article first appeared on TODAY.com. Read more from TODAY here:
]]>Moderna on Monday said its combination vaccine that targets both Covid-19 and the flu was more effective than existing standalone shots for those viruses in a late-stage trial.
The biotech company is the first to release positive phase three data on a Covid and flu combination shot, giving it a potential lead over rival vaccine makers Pfizer and Novavax.
Moderna plans to file for regulatory approval for its combination jab this summer in the U.S. and hopes it can enter the market in 2025, the company’s CEO Stephane Bancel said in an interview.
Moderna, Pfizer and Novavax have said that combination shots will simplify how people can protect themselves against respiratory viruses that typically surge around the same time of the year. The added convenience is critical as fewer Americans roll up their sleeves to get vaccinated against Covid.
Bancel added that combination shots could reduce the burden of respiratory viruses on pharmacists and the broader U.S. health-care system, which has been grappling with a labor shortage that has many workers stretched thin.
Moderna’s messenger RNA combination shot, called mRNA-1083, is made up of both the company’s vaccine candidate for seasonal influenza and a newer, “next-generation” version of its Covid shot. Both of those experimental vaccines – mRNA-1010 and mRNA-1283 – have shown positive results in separate phase three trials.
The ongoing late-stage trial on mRNA-1083 examined the combination shot in 8,000 patients.
The study compared the combination shot with an enhanced flu vaccine called Fluzone HD and Moderna’s currently licensed Covid shot, Spikevax, in one group of patients ages 65 and above. The trial also compared Moderna’s combination jab with a standard flu shot called Fluarix and Spikevax in another group of participants between the ages of 50 and 64.
In both age groups, a single dose of Moderna’s combination vaccine produced “statistically significantly higher” immune responses against three strains of influenza and the Covid omicron variant XBB.1.5.
Moderna said the safety of the combination shot, along with how well patients could tolerate it, was acceptable. The most common side effects were injection site pain, fatigue, muscle pain and headache. The majority of those effects were mild to moderate in severity.
Moderna is also developing a combination shot targeting the flu and RSV, and another vaccine targeting all three respiratory viruses: Covid, flu and RSV.
Meanwhile, Pfizer and BioNTech also are studying a vaccine that targets both Covid and the flu in a late-stage trial. Novavax is developing a combination for those viruses as well, but its Covid shot uses protein-based technology.
]]>The concept, designed to quickly convey health ramifications to busy consumers about the food and beverages they are considering purchasing, is not novel: Worldwide, dozens of countries already have front-of-package nutrition labels that come in various designs. In Chile, for example, a stop sign symbol on the front of an item indicates if it has high sugar, saturated fat, sodium or calories. In Israel, there’s a red warning label on such food and drinks. And in Singapore, beverages display a letter grade based on how nutritious they are.

Advocates have been asking the FDA for nearly two decades to require front-of-pack labels, which they say help people make healthier choices and prod food manufacturers to reformulate their recipes so they have fewer warnings on their products. The FDA stayed largely silent on the issue until it announced intentions to explore front-of-pack labels as part of a national health strategy released during a landmark White House Conference on Hunger, Nutrition, and Health in 2022. Since then, it has reviewed literature on front-of-pack labeling and conducted focus groups to test designs for labels.
But the idea faces opposition from trade associations representing America’s food and beverage makers, who created their own voluntary system for highlighting certain nutrients on the front of packages over a decade ago. And some of the label designs being considered by the FDA could be challenged on First Amendment grounds.
“The U.S. does interpret free speech much broader and more inclusive of corporate speech than any other country in the world,” said Jennifer Pomeranz, an associate professor at the New York University School of Global Public Health who has researched First Amendment obstacles to mandating front-of-package food labels.

Designs that are purely factual — stating the number of grams of added sugars, for example — are more likely to be considered constitutional than interpretive designs that have shapes or colors that characterize a product as unhealthy, her research found.
“It starts to get more iffy when you go into subjective,” Pomeranz said.
Among the multiple label options tested by the FDA, some used traffic light colors to indicate whether there was a high (red), medium (yellow) or low (green) amount of saturated fat, sodium or added sugars; others stated if a product was “high in” those nutrients, sometimes adding the percentage of the recommended daily value that a serving size contains.
A spokesperson for the FDA declined to disclose to NBC News which label design it will use and did not say exactly when the agency will release its proposed rule, other than to say “it is targeting for this summer,” despite previously setting a deadline of this month.
The Consumer Brands Association and the food industry association FMI, which created a voluntary label system for the food and beverage industry called Facts up Front that launched in 2011, have made clear they are against mandatory interpretive designs such as a red light/green light system. Interpretive labels “will raise unnecessary fear in consumers based on a single limiter nutrient without providing meaningful information as to how that food item might fit into overall healthy eating patterns,” they wrote in a public comment to the FDA in 2022.
They also say their voluntary system addresses the needs of consumers. Facts up Front uses up to four icons on the front of packages to highlight calories, saturated fat, sodium and added sugars per serving size. Manufacturers can also include nutrition information for up to two “nutrients to encourage,” such as potassium or fiber. The Consumer Brands Association says hundreds of thousands of products carry Facts up Front: 207,000 foods and beverages displayed them as of 2021, according to the most recent data available from the group.
“It’s really giving consumers a quick, consistent and holistic look at what the nutrition composition is of whatever they’re purchasing, and then helping those consumers make informed decisions,” said Sarah Gallo, the association’s vice president of product policy.
Advocates for mandatory front-of-package labeling disagree, arguing that the Facts up Front campaign is not used enough: By contrast, the nutrition facts label that is federally mandated to be on the backs or sides of packages appears on billions of products.
“Front-of-pack labeling is only reliable for consumers if it appears across the entire food supply, not only on the products of a handful of manufacturers who opt into a voluntary program,” said Eva Greenthal, a senior policy scientist at the food and health advocacy group Center for Science in the Public Interest, which first petitioned the FDA in 2006 to implement front-of-pack labels.
She also said Facts up Front does not give enough context to be helpful.
“Facts up Front does not provide any additional tools to help the consumer interpret that information,” she said. “We need something like the word ‘high in.’”
Courtney Gaine, president and CEO for the Sugar Association, the trade association for the U.S. sugar industry, said her group supports transparency but questions whether mandatory front-of-pack labeling will improve Americans’ diets.
“It just doesn’t seem like this has the evidence to show that this will make a difference,” she said.
But Greenthal and other advocates say there’s data from around the world to support it. In Chile, which in 2016 became the first country to apply front-of-package nutrition information, studies show people have made healthier consumer purchases and are choosing from healthier product reformulations.
“I think it’s a very classic food industry, anti-regulatory tactic to deny the science supporting a new policy that might be difficult to implement but is beneficial for society,” Greenthal said.
In its own review of scientific literature on front-of-pack labels, the FDA concluded that the labels “can help consumers identify healthy foods” and “appear helpful for those with lower nutrition knowledge and busy shoppers.”
The discussion comes as the percentage of Americans who are considered overweight or obese has risen, with obesity affecting about 42% of U.S. adults. More than 1 million Americans die from diet-related diseases such as cardiovascular disease, diabetes and certain cancers annually, according to the FDA.
The statistics don’t mean that the nutrition facts box that became required on the backs or sides of food packaging three decades ago has been a failure, said Xaq Frohlich, an associate professor of history at Auburn University and the author of the book “From Label to Table: Regulating Food in America in the Information Age.”
“Every time there’s been a change in the label, the food industry has reformulated its foods,” he said. “So even if you’re not reading the label, the food is changing, and it’s having that kind of impact.”
Greenthal said there are many people who would benefit from more nutritional information on the front of packages: busy parents rushing through the supermarket, people with low levels of nutrition literacy, and anyone else with limited time and energy to invest in their food choices.
“Policies like front-of-package labeling couldn’t come any sooner,” she said. “Diet-related chronic disease is one of the most important problems facing our country and hindering our population’s health.”
This story first appeared on NBCNews.com. More from NBC News:
]]>“I didn’t think much of it,” Salvador, now 21 of New Orleans, tells TODAY.com. “It was just getting bigger.”
As it grew, he worried that the mass was a sign something was seriously wrong. In the fall, he asked his mom about it, and she examined it. Concerned, she took Salvador to a hospital, and he eventually learned that he had stage 1 testicular cancer. He’s sharing his story so other young people with cancer feel less alone.
“Maybe someone will relate to it,” Salvador says. “Maybe somebody will find hope in my story.”
A lump that keeps growing
Over the summer of 2020, Salvador noticed the bump but thought his body had just changed.

“I thought it was normal,” he says. Then it began growing, and he became worried. In October, he mentioned it to his mom, who believed they should visit the hospital.
“They did some scans. They did some checks,” Salvador recalls. “They said, ‘Yes, this is cancerous.’”
The doctors recommended removing both testicles, but Salvador’s mother balked at this. She hoped to someday have grandchildren and thought that this plan was too aggressive for her teen son. The two visited a doctor at Children’s Hospital of New Orleans for a second opinion. Doctors there shared some welcome news.
“They were like, ‘OK, we’re going to do the best to save one (testicle),’” Salvador recalls. “But the other one definitely has to come out.”
The doctor removed the testicle and several lymph nodes during surgery and diagnosed Salvador with stage 1 rhabdomyosarcoma, a type of soft tissue cancer that can occur in connective tissue or muscle, according to the National Cancer Institute.
“The biggest surgery was the one where they took out my lymph nodes,” he says. “They opened up the whole chest area, stomach area.”
Following that surgery, Salvador underwent radiation for a month and then eight months of chemotherapy with infusions once a week. Treatment felt tough at times.
“I lost my hair. I was nauseous,” he says. “I was pretty weak.”
Following the completion of chemotherapy, Salvador was cancer free. He had another surgery where they gave him a prosthetic testicle. While going through cancer treatment as a teen felt difficult, he was able to enjoy his final year in high school.
“(My) hair grew back,” he says. “My late senior year, everything went back to normal.”
Testicular cancer
While Salvador’s cancer grew in his testicle, it’s not the same type of cancer often associated with testicular cancer diagnosis, such as the type that Lance Armstrong had, Dr. Pinki Prasad, oncologist and hematologist at Children’s Hospital New Orleans and one of Salvador’s doctors, tells TODAY.com.
“Ronal actually had a type of sarcoma, rhabdomyosarcoma, that can be found very often in the … testicular region,” she explains, adding that it’s more common in children than adults.

This type of cancer doesn’t have many noticeable symptoms other than a lump on the testicles — “usually painless, but it’s a bump that gets bigger with time and doesn’t get better,” Prasad says. “Sometimes it will be painful, and that’s what brings this to (their) attention.”
She estimates that, in 90% of cases of testicular rhabdomyosarcoma, a lump is the only sign. Prasad adds that “very rarely do we see pain with urination, blood in the urine.”
While Prasad says all pediatric cancers are considered rare, including testicular rhabdomyosarcoma, she urges boys to be aware of their bodies and say something if they notice any changes.
“Once they hit puberty, they should be checking their testicles at least once a month,” she says. “No one is going to know outside of them if there’s any changes, and so it’s really important for them to get used to knowing what’s normal for them.”
Treatment for testicular rhabdomyosarcoma includes surgery to remove the testicle with the cancer and lymph nodes, which can be followed by radiation and chemotherapy.
Like any cancer, patients diagnosed with rhabdomyosarcoma in early stages have good outcomes with lower risk of recurrence. Still, Prasad says doctors closely monitor people for several years.
“They resume their normal lives pretty quickly. They go back to school. They do all the things they want to do,” she says. “We do follow them for a very indefinite amount of time, and most of these patients are survivors.”
With testicular cancer, people often feel hesitant to share symptoms with their family or doctor.
“There is a stigma,” Prasad says. “Most of these patients who have some sort of a testicular tumor do end up having a testicle removed, which can lead to some body issues.”
Prasad notes that prosthetic testicles are available, and more than half of her patients opt to have one.

College and beyond
For years, Salvador hoped to become a doctor. After graduating from high school, he started college and is studying pre-med.
“I want to be a cardiologist,” he says. “When I was younger, I used to have a lot of heart issues, and I used to always see cardiologists. They always looked so happy.”
This summer, Salvador plans to work at his family’s business and read for fun. He hopes his story encourages others to be aware of their health.
“It’s important for everybody to learn about their bodies, how to examine it on their own,” he says. “It’s important to know how to take care of yourself.”
This article first appeared on TODAY.com. Read more from TODAY here:
]]>The drug, called medetomidine, is linked to a recent spate of deadly overdoses throughout the Midwest and Northeast. According to NBC News it dramatically slows down breathing, heart rate, blood pressure and decreases activity in the brain and spinal cord. It’s not meant for use in people.
“It’s really concerning, the types of contaminants that we are seeing,” said Dr. Natasha Bagdasarian, chief medical executive of the Michigan Department of Health and Human Services. “Drugs are becoming deadlier.”
Medetomidine is more potent than a similar animal sedative, xylazine, or “tranq,” that’s become widespread in the U.S. over the past several years.
NPS Discovery, a group that researches illegal drugs, reported finding medetomidine in Maryland as early as July 2022.
It’s clear that the drug is now moving west: It was found during toxicology tests in three people in Michigan who died of drug overdoses, state health officials said Thursday. The cases occurred in separate parts of the state and are not linked.
Last month, health officials in Chicago linked medetomidine to an increase in overdoses — the first time it’d been detected in the city. The sedative was found in combination with opioids such as fentanyl, nitazenes and heroin, as well as with tranq and the anti-anxiety drug alprazolam (Xanax).
The Philadelphia Department of Public Health also reported in May that medetomidine had arrived in the city. It showed up last month in Pittsburgh, too.
People who take the drug can remain sedated for at least three hours, according to Philadelphia’s health alert.
Sporadic reports of the drug are expected to become more widespread as the drug continues to make its way nationwide, said Linda Cottler, director of the National Drug Early Warning System, which monitors emerging drug use trends.
Cottler’s team hasn’t seen rampant signs of medetomidine — yet.
“It’s like a drip, drip, drip until it kind of explodes,” said Cottler. “This is the way drugs travel.”
The rise of medetomidine comes as overdose deaths have fallen slightly. More than 107,000 people died of a drug overdose last year, down from about 111,000 in 2022, according to a recent report.
Medetomidine is particularly concerning because its effects can’t be reversed by drugs like Narcan, also called naloxone. And there are no test strips that can detect it.
“It does seem like the new trend is adding sedatives or tranquilizers and other types of nonopioid drugs to fentanyl, which makes opioid reversal much more complicated,” said Joseph Palamar, an associate professor in the section on tobacco, alcohol and drug use at NYU Langone in New York City, and deputy director of the National Drug Early Warning System. “How are you going to reverse an overdose with naloxone if this keeps you sedated?”
Still, experts continue to urge people to use naloxone in cases of overdose.
“Even though there is not a specific way to reverse medetomidine, we know that it has been found in conjunction with opioids like fentanyl,” Bagdasarian said. “Our primary goal here is to prevent overdose deaths, and as the drug supply becomes deadlier, we have to become more innovative and try to step a step ahead of the problem.”
This story first appeared on NBCNews.com. More from NBC News:
]]>Researchers led by the Cleveland Clinic linked the low-calorie sugar substitute xylitol to an increased risk of heart attack, stroke or cardiovascular-related deaths, according to a study published today in the European Heart Journal.
Xylitol is a sugar alcohol that is found in small amounts in fruit and vegetables, and the human body also produces it. As an additive, it looks and tastes like sugar but has 40% fewer calories. It is used, at much higher concentrations than found in nature, in sugar-free gum, candies, toothpaste and baked goods. It can also be found in products labeled “keto-friendly,” particularly in Europe.
The same research team found a similar association last year to the popular sugar substitute erythritol. The use of sugar substitutes has increased significantly over the past decade as concerns about rising obesity rates mount.
“We’re throwing this stuff into our food pyramid, and the very people who are most likely to be consuming it are the ones who are most likely to be at risk” of heart attack and stroke, such as people with diabetes, said lead author Dr. Stanely Hazen, chair of cardiovascular and metabolic sciences at Cleveland Clinic’s Lerner Research Institute.
Many heart attacks and strokes occur in people who do not have known risk factors, like diabetes, high blood pressure or elevated cholesterol levels. The research team began studying sugar alcohols found naturally in the human body to see if the compounds might predict cardiovascular risk in these people.
In the study, the investigators measured the level of naturally occurring xylitol in the blood of more than 3,000 participants after overnight fasting. They found that people whose xylitol levels put them in the top 25% of the study group had approximately double the risk for heart attack, stroke or death over the next three years compared to people in the bottom quarter.
The researchers also wanted to understand the mechanism at work, so they fed xylitol to mice, added it to blood and plasma in a lab and gave a xylitol-containing drink to 10 healthy volunteers. In all these cases, xylitol seemed to activate platelets, which are the blood component that controls clotting, said Hazen. Blood clots are the leading cause of heart attack and stroke.
“All it takes is xylitol to interact with platelets alone for a very brief period of time, a matter of minutes, and the platelet becomes supercharged and much more prone to clot,” Hazen said.
The next question is what causes naturally-occurring xylitol to be elevated in some people and how do you lower it, said Dr. Sadiya Khan, a cardiologist at Northwestern Medicine Bluhm Cardiovascular Institute and a professor of cardiovascular epidemiology at Northwestern Feinberg School of Medicine who was not involved in the new study.
Much more research needs to be done, said Hazen. In the meantime, he is telling patients to avoid eating xylitol and other sugar alcohols, whose spelling all end in ‘itol.’ Instead, he recommends using modest amounts of sugar, honey or fruit to sweeten food, adding that toothpaste and one stick of gum are probably not a problem because so little xylitol is ingested.
The report had key limitations.
First, the study of naturally occurring xylitol in people’s blood was observational and can show only an association between the sugar alcohol and heart risk. It does not show that xylitol caused the higher incidence of heart attack, stroke or death.
Nevertheless, given the totality of the evidence presented in the paper, “it’s probably reasonable to limit intake of artificial sweeteners,” said Khan. “Perhaps the answer isn’t replacing sugar with artificial sweeteners but thinking about more high quality dietary components, like vegetables and fruits, as natural sugars.”
Artificial sweeteners shouldn’t be difficult to avoid, said Joanne Slavin, PhD, RDN, a professor of food science and nutrition at the University of Minnesota-Twin Cities. They are listed on the ingredient list of packaged goods.
“Would I say never eat xylitol?” asked Slavin, who had no connection to the study. For some people who struggle to reduce sugar in their diet, sugar substitutes are one tool, and it comes down to personal choice, she said.
While Slavin found the study interesting and cause for some concern, she noted that sugar alcohols are expensive and are generally used in very small amounts in gum and sugar-free candies.
Another limitation of the study is that the participants whose xylitol levels in the blood were measured were at high risk for or had documented heart disease, and so the results may not apply to healthy individuals.
Still, many people in the general public share the characteristics of the study participants, said Hazen.
“In middle-aged or older America, it’s common to have obesity and diabetes or high cholesterol or high blood pressure,” he said.
This article first appeared on NBCNews.com. Read more from NBC News:
]]>The FDA first ordered the company to stop selling its products in 2022, but they stayed on shelves pending an appeal. Juul has maintained its status as the No. 2 e-cigarette maker in the U.S. during this time.
Now, the FDA says Juul’s products are back under agency review — although it emphasized that this new status was not an indication they would be fully cleared.
It said federal statutes barred it from disclosing additional information.
In a statement, Juul said it appreciated the FDA’s decision, adding it now looks forward to “re-engaging with the agency on a science- and evidence-based process to pursue a marketing authorization” for its products.
“We remain confident in the quality and substance of our applications and believe that a full review of the science and evidence will demonstrate that our products meet the statutory standard of being appropriate for the protection of public health,” the company said.
Even as Juul has pursued its appeal of the 2022 ban, that initial FDA ruling significantly disrupted the company’s finances, prompting a bailout from two of its largest investors, The Wall Street Journal has reported.
To date, the FDA has given only 23 e-cigarette products, made by just three companies, official approval to be marketed to consumers.
This story first appeared on NBCNews.com. More from NBC News:
]]>The WHO said it wasn’t clear how the man became infected, although H5N2 has been reported in poultry in Mexico.
There are numerous types of bird flu. H5N2 is not the same strain that has infected multiple dairy cow herds in the U.S. That strain is called H5N1 and three farmworkers have gotten mild infections.
Other bird flu varieties have killed people across the world in previous years, including 18 people in China during an outbreak of H5N6 in 2021, according to a timeline of bird flu outbreaks from the U.S. Centers for Disease Control and Prevention.
Mexican health officials alerted the WHO that a 59-year-old man who died in a Mexico City hospital had the virus despite no known exposure to poultry or other animals.
According to family members, the WHO release said, the patient had been bedridden for unrelated reasons before developing a fever, shortness of breath and diarrhea on April 17. Mexico’s public health department said in a statement that he had underlying ailments, including chronic kidney failure, diabetes and high blood pressure.
Hospital care was sought on April 24 and the man died the same day.
Initial tests showed an unidentified type of flu that subsequent weeks of lab testing confirmed was H5N2.
The WHO said the risk to people in Mexico is low, and that no further human cases have been discovered so far despite testing people who came in contact with the deceased at home and in the hospital.
There had been three poultry outbreaks of H5N2 in nearby parts of Mexico in March but authorities haven’t been able to find a connection. Mexican officials also are monitoring birds near a shallow lake on the outskirts of Mexico City.
Whenever bird flu circulates in poultry, there is a risk that people in close contact with flocks can become infected. Health authorities are closely watching for any signs that the viruses are evolving to spread easily from person to person, and experts are concerned as more mammal species contract bird flu viruses.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
]]>The Centers for Disease Control and Prevention said it has received reports of 162 people who fell ill as a result of the salmonella strain tied to cucumbers, with 54 of those individuals hospitalized. In the tri-state, New York has reported 19 people sick, while there have been three in New Jersey and two in Connecticut, the CDC found.
Pennsylvania has had the most cases reported, with 27 people falling ill.
No deaths have been reported as a result of the salmonella poisonings, according to the CDC.
Earlier in the week, the U.S. Food and Drug Administration recalled whole cucumbers shipped to 14 states by Fresh Start Produce, a Florida-based company, from May 17-21. New York, New Jersey and Pennsylvania were among the states where the cucumbers were sent.
The FDA and CDC were conducting further tests on a wider sample of cucumbers to see if the same strain of salmonella is causing the wider outbreak.
Fresh Start Produce said the affected produce was “unlikely” to have made it to store shelves, though customers should check with their places of purchase to determine whether the recall impacts their produce. The recalled cucumbers should no longer be in stores, the CDC said, and the recall did not cover English or mini cucumbers.
Salmonella symptoms
Those who infected with the salmonella bacteria will likely experience diarrhea, fever and stomach cramps. Symptoms will typically begin from six hours to six days after ingesting the bacteria, according to the CDC. Most people recover without any special treatment after four to seven days.
Some people, especially those under the age of 5 or over the age of 65, or with weakened immune systems, could experience more severe illnesses that could call for medical treatment or hospitalization.
]]>Testing detected salmonella in a cucumber distributed by Fresh Start Produce, of Delray Beach, Florida, which last week recalled whole cucumbers shipped to certain states from May 17 to May 21, according to the Centers for Disease Control and Prevention. Further testing is underway to see if that strain of salmonella is causing the outbreak. The produce should no longer be available in stores.
The CDC received reports of 162 people sickened with salmonella potentially tied to the cucumbers in 25 states and Washington, D.C., between March 11 and May 16. At least 54 people were hospitalized, the agency said. No deaths were reported.
Consumers should not eat recalled cucumbers. People who bought cucumbers recently should check with the store where they purchased them to see if they’re part of the recall. Wash items and surfaces that may have been in contact with the produce using hot, soapy water or a dishwasher.
The CDC and the U.S. Food and Drug Administration also are investigating an outbreak of a second type of salmonella that has sickened at least 158 people in nearly two dozen states to see whether it’s connected to the same food. The outbreaks share several similarities, the agencies said.
Salmonella can cause symptoms that begin six hours to six days after ingesting the bacteria and include diarrhea, fever and stomach cramps. Most people recover without treatment within a week, but young children, people older than 65 and those with weakened immune systems can become seriously ill.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
]]>The case report, published Wednesday in JAMA Dermatology by doctors at NYU Langone Health in New York City, comes as clinicians worldwide increasingly say they’re having trouble treating fungal infections.
“We think a lot about antibacterial resistance, but this is a very important time for us to think about anti-fungus resistance,” said Mahmoud Ghannoum, a professor of dermatology at the Case Western Reserve University School of Medicine in Cleveland. He was not involved with the new report.
The new case involves a New York City man in his 30s who reported having sex with multiple men during a trip to England, Greece and California. When he got home, he developed a red, itchy rash on his legs and across his groin and buttocks.
Tests revealed he had a sexually transmitted fungus, called Trichophyton mentagrophytes type VII. It is the first time the fungus has been identified in the U.S. Last year, doctors in France reported 13 such cases. Twelve of those patients were men who have sex with men.
The American man’s infection responded to standard anti-fungal medications but ultimately took four and a half months to heal fully.
He was put on fluconazole for four weeks without improvement, then six weeks of terbinafine and approximately eight additional weeks of itraconazole. All are oral anti-fungals.
He did not have any other infections that could have made the problem worse.
Dr. Avrom Caplan, an assistant professor of dermatology at the NYU Grossman School of Medicine and author of the new report, said the case should raise awareness but not cause alarm in the general public.
“There’s no evidence that this is widespread, or that this is something that people really need to be worried about,” he said. “But if people are having itchy eruptions in areas like the groin, and it’s not getting better, see a doctor.”
Rash may look like eczema
While the infection was most likely transmitted through sexual contact, Caplan couldn’t rule out the possibility that the man acquired the fungus at a sauna he’d visited two months prior to his symptoms. The man said his sexual partners did not show any signs of ringworm.
Caplan said the rash may look more like an eczema flare than typical ringworm infections that form in circles. The infection is not life-threatening, but can cause permanent scarring.
He previously identified the first two cases of a different ringworm infection in 2023. Those infections, caused by Trichophyton indotineae, are not considered STIs but are drug-resistant and highly contagious.
Since then, Caplan’s team at NYU Langone Health has identified a total of 11 cases of Trichophyton indotineae ringworm in both men and women in New York City.
The new case report is “notable” said Jeremy Gold, a medical epidemiologist at the Centers for Disease Control and Prevention. He stressed that doctors should consider fungi along with viruses and bacteria as a potential cause of sexually transmitted disease.
“Oftentimes, what happens is that these patients receive multiple courses of antibacterial drugs which are not going to make the fungus better,” he said. “Clinicians should keep this in mind so that patients can get appropriate care.” He was not involved in the new case report.
Caplan also encouraged people to speak up and seek out treatment. For now, Trichophyton mentagrophytes type VII is treatable.
“If you have a rash or lesions on your skin that aren’t getting better, and you think it might be ringworm,” he said, “see your doctor.”
This story first appeared on NBCNews.com. More from NBC News:
]]>A panel of advisers to the Food and Drug Administration voted 10-1 against the overall benefits of MDMA when used to treat post-traumatic stress disorder. They cited flawed study data, questionable research conduct and significant drug risks, including the potential for heart problems, injury and abuse.
“It seems like there are so many problems with the data — each one alone might be OK, but when you pile them on top of each other … there’s just a lot of questions I would have about how effective the treatment is,” said Dr. Melissa Decker Barone, a psychologist with the Department of Veterans Affairs.
The FDA is not required to follow the group’s advice and is expected to make its final decision by August, but the negative opinion could strengthen FDA’s rationale for rejecting the treatment.
MDMA is the first in a series of psychedelics — including LSD and psilocybin — that are expected to come before the FDA for review in the next few years as part of a resurgence of interest into the drugs’ medical potential, which advocates claim could transform the treatment of mental health disorders.
But FDA advisers spent most of Tuesday’s meeting leveling pointed questions and criticisms at the research submitted on MDMA, which is sometimes called ecstasy or molly. Panelists pointed to flawed studies that could have skewed the results, missing follow-up data on patient outcomes and a lack of diversity among participants. The vast majority of patients studied were white, with only five Black patients receiving MDMA, raising questions about the generalizability of the results.
“The fact that this study has so many white participants is problematic because I don’t want something to roll out that only helps this one group,” said Elizabeth Joniak-Grant, the group’s patient representative.
The FDA advisers also drew attention to allegations of misconduct in the trials that have recently surfaced in news stories and a report by the nonprofit Institute for Clinical and Economic Review, which evaluates experimental drug treatments. The incidents include a 2018 report of apparent sexual misconduct by a therapist interacting with a patient.
Lykos Therapeutics, the company behind the study, said it previously reported the incident to the FDA and regulators in Canada, where the therapist is based.
Lykos is essentially a corporate spinoff of the nation’s leading psychedelic advocacy group, the Multidisciplinary Association for Psychedelic Studies, or MAPS, which funded the studies. The group was founded in 1986 to promote the benefits of MDMA and other mind-altering substances.
Lykos said in a statement after the meeting that it would work with regulators to address the panel’s concerns.
“While we are disappointed in the vote, we are committed to continuing to collaborate with the FDA with their ongoing review of our (drug application) over the coming weeks,” the company stated. FDA is expected to issue its decision by Aug. 11.
The overwhelmingly negative panel ruling could further derail financial investments in the fledgling psychedelic industry, which has mainly been funded by a small number of wealthy backers. Dozens of startup companies have launched in recent years seeking to study psilocybin, ketamine and others drugs for conditions like depression and addiction, though many have struggled to raise money.
MDMA doesn’t cause the visual hallucinations commonly associated with psychedelics. Instead, its main effect is triggering feelings of intimacy, connection and euphoria. When used to enhance talk therapy, the drug appears to help patients process their trauma and let go of disturbing thoughts and memories.
But the panel struggled with the reliability of the results reported by Lykos, given the difficulties of objectively testing psychedelic drugs.
Because MDMA causes intense, psychological experiences, almost all patients in two key studies of the drug were able to guess whether they had received the MDMA or a dummy pill. That’s the opposite of the approach generally required for high-quality drug research, in which bias is minimized by “blinding” patients and researchers to whether they received the drug under investigation.
“I’m not convinced at all that this drug is effective based on the data I saw,” said Dr. Rajesh Narendran, a University of Pittsburgh psychiatrist who chaired the panel.
Panelists also noted the difficulty of knowing how much of patients’ improvement came from MDMA versus simply undergoing the extensive therapy, which totaled more than 80 hours for many patients. Results were further marred by other complicating factors, including a large number of patients who had previously used MDMA or other psychedelics drugs recreationally.
Nearly three dozen public speakers addressed the panel during a comment period, including veterans who said they benefitted from MDMA therapy, medical professionals who advised against its use and journalists and independent researchers who detailed the allegations of misconduct in the trials.
The meeting concluded with several experts encouraging Lykos and the FDA to continue studying psychedelics for PTSD, citing the field’s potential to help patients.
“I think this is a really exciting treatment and I’m encouraged by the results to date,” said Dr. Paul Holtzheimer of the VA’s National Center for PTSD, “but from a safety and efficacy standpoint I feel it’s still premature.”
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
]]>“It wasn’t a terribly hard workout,” the 49-year-old from Avon Lake, Ohio, recalls to TODAY.com. “But after, my triceps and my biceps and my chest were all incredibly sore.”
Worried, Prewitt’s trainer brought over a sports medicine doctor, who advised Prewitt to go to urgent care. His wife, Meredith, packed their kids in the car and rushed to pick him up. She missed the exit for urgent care but noticed they were right by a hospital. She glanced at her husband, who was gray and cold, and rushed him to the emergency room. This was fortunate as Prewitt was having a heart attack.
“I had one single artery that was completely blocked,” he says. “The artery is the left anterior descending artery … the widow maker.”
‘Picture of health’
Over the years, Prewitt enjoyed exercise, running hundreds of 5Ks, two marathons and participating in CrossFit. He doesn’t drink or use drugs, and eats a healthy diet and prioritizes sleep.
“I am often thought of as a picture of health,” he says. “I do all the things you’re supposed to do.”

For the year leading up to the heart attack, Prewitt felt good for the most part, but he did notice that he was easily fatigued. He chalked it up to his age and parenthood.
“I didn’t know if this is what (nearing) 50 (felt) like,” Prewitt says. “I’ve got two young boys who are 9 and 6, and was finding myself at the end of my workday a little tired and sometimes having to need a break before we go play baseball or kick a soccer ball around the yard.”
Just to be safe, he visited his doctor and had bloodwork done three times. The most recent was a month before his heart attack.
“I had no markers of any kind. My total cholesterol seemed to be OK,” he says. “I had no real health issues other than being tired.”
For Christmas, his wife bought him a five-pack of sessions with a trainer, and Prewitt scheduled his first one immediately. After the workout, he explained the sensation he felt in his arms and chest to the trainer, who felt worried and found a doctor. That doctor recommended that Prewitt have someone drive him to an urgent care to get his heart checked.
“I call my wife to come pick me up, which in and of itself is a miracle. I’m pretty bullheaded,” he says. “I’m not one to take advice.”
On the way to the clinic, they got lost.
“She missed the exit for the urgent care and gets off the highway, and I’m slumped over in my seat,” Prewitt explains. “She’d never seen anyone this gray, and she grabbed my hand. It was ice cold. My heart had stopped.”
Across the street, though, Meredith saw Cleveland Clinic’s Avon Hospital at Richard E. Jacobs campus.
“She ran three red lights and pulled over to the ER and ran inside,” he says. “A bunch of health care workers came out, pulled me out of the car, brought me back to work on me.”
For 20 minutes, Prewitt received CPR and a dozen defibrillator shocks as the staff tried to get his heart back into a steady rhythm. After they stabilized him, they sent him to another Cleveland Clinic facility with a cardiac catheterization laboratory. Testing showed that Prewitt had a widow maker heart attack.
“I was able to be brought back,” Prewitt says, adding that he regained consciousness about 24 hours later.
Widow maker heart attack
When the left anterior artery has a blockage, what’s known as a widow maker heart attack can occur. In younger patients like Prewitt, there might not be any symptoms.
“About 30% of patients don’t have any previous symptoms, but they suddenly drop dead,” Dr. Emad Nukta, a cardiologist at Cleveland Clinic, tells TODAY.com. “We see that mostly with the younger patients rather than the older patients, and that’s where the term of widow maker comes from.”

Sometimes patients have subtle symptoms, such as fatigue, that they might not think of as a sign of something being wrong.
“The worst one is the one where they really did not have symptoms. They were not aware of anything,” Nukta says. “They were exercising or working out, lifting weights or on a treadmill, and suddenly they drop dead.”
Symptoms can include:
- A feeling like indigestion
- Arm or neck pain
- Chest pain
Nukta says often a heart attack comes with extreme pain, but that’s not always the case.
“There’s a major misconception. They think pain is something like real burning or severe pain,” he says. “But chest pain is really not that severe pain. It’s uneasy pressure like pain, squeezing pain.”
People with lifestyle behaviors that increase the risks of their arteries hardening, called atherosclerosis, are at greater risk of experiencing a widow maker, Nukta says. Risk factors include:
- Being a smoker
- Having high blood pressure
- Having high cholesterol
- Having a family history of heart attack
- Having diabetes
He says it’s important for patients to know their family history to understand if they’re at risk of a widow maker. After having a heart attack, most patients need to continue seeing a cardiologist.
“I will tell my patients if you have a cardiac event, you’re stuck with me,” he says. “You will always be a heart patient.”
‘A very blessed life’
While Prewitt knew that one of his grandfathers underwent two bypass surgeries, he did not realize his other grandfather died at age 49 of a widow maker heart attack.
“My lesson to all the people I’ve told this to is that you might think that you’re doing all the right things, but if you are unaware of your genetic history, you need to find out and also let your general practitioner know,” he says.

Prewitt takes several medications now to support his heart and keep his artery open. He recently finished cardiac rehabilitation.
“By the end of 12 weeks, I was running nine-minute miles for a good distance,” he says. “I have no restrictions. A month ago, I was in Universal (Studios) with my wife and my boys and rode some rollercoasters. I continue to run and lift weights.”
This experience has taught Prewitt the importance of healthy habits, and he encourages others to “take their health very seriously.”
“There are health markers or genetic things that are coded in you that you cannot run away from,” he says. “Through knowledge and proactive medical care, you can identify these things early enough and be able to do something about it before it becomes a real event.”
Having a heart attack caused Prewitt to re-examine his life.
“It’s incredibly painful to think about my young boys going through life without their father,” he says. “For the majority of my life, I’ve been terrified of dying. Have I done enough? Have I lived enough, lived the type of life that I want to? And after this event I am not fearful of dying anymore. … I’ve lived a very blessed life.”
This story first appeared on TODAY.com. More from TODAY:
]]>“It was basically like a hand sanitizer solution,” Whitehead shared with NBC News, who lives in Torrance, California. “Smelled like hand sanitizer, looked like hand sanitizer.”
The gel wasn’t hand sanitizer, though. It was a hormonal solution meant to block Whitehead’s sperm production. The gel was male birth control.
Until this past winter when his participation concluded, Whitehead was a volunteer in a phase 2 trial for the gel. The product — which contains testosterone and a synthetic hormone called Nestorone that reduces sperm production — is the most advanced among a crop of novel birth control options for men.
If the Food and Drug Administration approves the gel, Whitehead said he would definitely keep using it, especially after watching his partner struggle with available female birth control options.
“The gel was such an easy process,” he said. “It was basically like taking the pill for the day.”
Whitehead said he didn’t notice side effects using the gel beyond some upper back acne and possibly a bit of weight gain, although that could have been linked to a new sedentary job.
Hormonal gel trial shows promise
On Sunday, at the Endocrine Society’s conference in Boston, researchers with the National Institutes of Health’s Contraceptive Development Program presented encouraging phase 2 trial results on the hormonal gel.
The trial involved 222 men, ages 18 to 50, who applied 5 milliliters of the gel (about a teaspoon) to each of their shoulder blades once per day.
The second part of the two-part trial is still underway. Initial findings showed that the contraceptive worked faster than expected, according to Diana Blithe, chief of NIH’s Contraceptive Development Program.
After 12 weeks of applying the gel every day, 86% of trial participants achieved sperm suppression, meaning they had only up to 1 million sperm per milliliter of semen, the amount the researchers deemed effective for contraception. On average, the timing for effective contraception was eight weeks.
In comparison, normal sperm counts without contraception can range from 15 million to 200 million per milliliter.
The faster-than-expected timing to suppress sperm is an encouraging sign, especially since past attempts have taken longer to reach these sperm levels, Blithe said in a news release about the new data.
Prior efforts using testosterone alone have required higher doses of the hormone, which can cause side effects. Because the gel includes both testosterone and Nestorone, it acts more quickly and requires less testosterone, she said.
Nestorone is a type of synthetic hormone called a progestin that’s already used in the vaginal ring contraceptive. Combining Nestorone and testosterone in the new gel is meant to keep men from producing sperm without affecting their sex drive or causing other side effects.
So far, the men in the gel clinical trial have shown low enough blood levels of testosterone to maintain their normal sexual function.
Researchers are now tracking how well the gel works to prevent pregnancy. Because of pregnancy risk, male participants are required to be in committed, monogamous relationships, and need consent from their female partners too. The couple must agree to use the gel as their only birth control and to have sex at least once a month for a year. Throughout the study, men have their sperm counts tested periodically, which is a good predictor of fertility. If the sperm counts remain low, the chances of pregnancy are slim.
After decades of early-stage attempts and failures, there are no federally approved male birth control drugs. Only a handful have even advanced into human trials.
It’s not because the approaches haven’t shown potential, researchers say, but because there hasn’t been enough funding or financial investment to complete expensive advanced human trials.
“We’ve been pushing for hormonal male contraceptives for 50 years, but there isn’t enough money available to really drive something through a very large phase 3 trial,” said Daniel Johnston, chief of the National Institute of Child Health and Human Development’s Contraception Research Branch.
If one male birth control drug gains approval from the FDA, pharmaceutical companies and industry investors would put more resources into other medications or products, Johnston believes.
“We’ve been chasing this for a long time,” Johnston said. “I hope we’re entering new territory.”
Nonhormonal options in development
Also at the Boston conference on Sunday, YourChoice Therapeutics said a very small trial in the U.K. — just 16 men — showed that its nonhormonal pill, YCT-529, was safe and free of side effects. The San Francisco company’s nonhormonal pill works by blocking the vitamin A receptor important for male fertility.
YourChoice is planning a larger trial, according to CEO Akash Bakshi.
“We’re excited to see what happens next,” Bakshi said.
Separately, a Charlottesville, Virginia, medical device company, Contraline, is developing a nonhormonal male birth control method that involves injecting a gel into the vas deferens, the tubes that transport sperm from the testicles.
Injecting the gel, called ADAM, involves a single, 15-minute procedure, said Kevin Eisenfrats, Contraline’s CEO and co-founder. Then, the gel is meant to stay in place for years. Contraline compares the long-acting reversible contraceptive to an intrauterine device (IUD) for women.
Contraline has been testing ADAM in an early clinical trial in Australia. In January, the company reported that among 25 clinical trial participants, the approach resulted in a 99.8% to 100% reduction in the number of motile sperm within 30 days of the procedure, Eisenfrats said.
“It’s honestly very similar to the experience patients have after a vasectomy,” he said. “Some of these patients had light bruising and swelling, which go away on their own.”
Contraline hopes to start testing ADAM in the U.S. in 2025.
Because Contraline is developing ADAM as a medical device and not a drug, it may be able to go through a speedier clinical trial and regulatory process than contraceptive drugs like the hormonal gel, experts suggest.
If it goes according to plan — which can be rare for novel products with no precedent — Eisenfrats said he’s aiming for an FDA approval in 2027.
Another company called Next Life Sciences is developing a similar method. Next Life’s approach, called Plan A, or Vasalgel, also involves blocking the vas deferens with a gel-like injection. Next Life is based in Flagstaff, Arizona. Next Life hasn’t started testing Plan A in people yet, although the company did test its method of injecting the gel in Canadian volunteers this past year.
Demand for new contraception is growing
U.S. and global surveys have found that men are willing to use contraception, said gynecologist Dr. Brian Nguyen, one of the investigators on the gel clinical trials.
“By and large, they always say they’d be interested,” said Nguyen, an associate professor of obstetrics and gynecology at the University of Southern California.
According to one 2023 survey published in the journal Contraception, three-quarters of 2,066 male respondents said they’d be willing to use new contraceptives.
In 2019, the nonprofit Male Contraceptive Initiative estimated more than 17 million men in the U.S. want more birth control options.
Heather Vahdat, the Male Contraceptive Initiative’s executive director, said interest in male birth control has been on the rise since the Supreme Court overturned Roe v. Wade in 2022.
Recent research from the University of Pittsburgh School of Public Health found that the number of young women and men choosing permanent birth control such as vasectomy and tubal ligation increased sharply after the court’s decision and has continued to rise.
A separate study conducted in part by the Male Contraceptive Initiative, showed that before the abortion ruling, 78% of men in the U.S. said they were interested in trying new birth control methods. Afterward, it climbed to 82%.
“The demand has always been there, but there’s a greater intensity now,” Vahdat said. “We get emails daily from people asking where they can sign up for clinical trials.”
Unlike a vasectomy, each of the new contraceptive approaches is meant to be reversible, so men can stop using them and regain their ability to have children.
“Vasectomies are a great solution for men who are done having kids,” Eisenfrats said. But reversing the procedure — which involves reattaching the vas deferens in a three-hour microsurgery — can be extremely challenging and doesn’t always work, he said.
While condoms can be highly effective against pregnancy or sexually transmitted infections when used perfectly, perfect use is hard to achieve. And condoms generally aren’t the preferred contraceptive in long-term relationships, YourChoice’s Bakshi said.
Why is male birth control taking so long?
The new trial results are encouraging to Vahdat. But she knows that it’s not enough to show a birth control method is safe and effective. The product needs substantial buy-in from investors, too.
“We have this classic line, ‘Male contraceptives have been 10 years away for 50 years,’” Vahdat said.
The reason, she believes, boils down to lack of funding.
After NIH research grants, the Male Contraceptive Initiative is the second-biggest funder of male contraceptive research in the world, according to Vahdat.
“That’s super exciting, except when you consider we only grant about $1.5 million a year,” she said.
On average, the Congressional Budget Office ballparks $1 billion to $2 billion as the amount needed to take a drug through clinical trials and onto the market.
Right now, there’s just not enough money to take any of these male birth control approaches through the FDA review process, USC’s Nguyen said.
The hormonal gel is the most advanced in clinical trials, but it still hasn’t gone through a much larger, lengthy phase 3 trial. As of now — in part due to funding uncertainties — plans to test the gel further are still up in the air.
Most academic researchers or small biotech companies developing new drugs rely on drugmakers with deep pockets to fund advanced trials. In exchange, these bigger companies usually expect a cut of profit once the drugs make it to market.
The studies so far have been funded by NIH and the independent nonprofit Lundquist Institute in Torrance, California.
As of now, Nguyen said, there’s no major pharmaceutical company stepping in to fund the male contraceptive gel’s next-stage trials.
“There has to be an industry partner,” he said.
Nguyen thinks the challenge is that despite evidence that men want options, many couples still depend on female birth control.
“But that doesn’t mean they are satisfied with them and wouldn’t appreciate a male method.” Nguyen said.
“People always ask, ‘How long will it be until we see this product on the market?’” Nguyen said. “Most people will say five to 10 years, but I disagree.”
However, the chance of any of these male contraceptives — the gel, the physical blockers, the pills and whichever new methods crop up next — making it to market depends on whether investors with deep pockets recognize the demand is really there, Vahdat said.
To reach this point, she believes, the conversation has to shift away from viewing male contraception and female contraception as mutually exclusive landscapes with two separate populations demanding them.
“I think of male contraception as women’s health,” she said. “You’re still preventing unintended pregnancy.”
This story first appeared on NBCNews.com. More from NBC News:
]]>It’s part of a long-term trend toward studying whether doing less — less surgery, less chemotherapy or less radiation — can help patients live longer and feel better. The latest studies involved ovarian and esophageal cancer and Hodgkin lymphoma.
Thirty years ago, cancer research was about doing more, not less. In one sobering example, women with advanced breast cancer were pushed to the brink of death with massive doses of chemotherapy and bone marrow transplants. The approach didn’t work any better than chemotherapy and patients suffered.
Now, in a quest to optimize cancer care, researchers are asking: “Do we need all that treatment that we have used in the past?”
It’s a question, “that should be asked over and over again,” said Dr. Tatjana Kolevska, medical director for the Kaiser Permanente National Cancer Excellence Program, who was not involved in the new research.
Often, doing less works because of improved drugs.
“The good news is that cancer treatment is not only becoming more effective, it’s becoming easier to tolerate and associated with less short-term and long-term complications,” said Dr. William G. Nelson of Johns Hopkins School of Medicine, who was also not involved in the new research.
Studies demonstrating the trend were discussed over the weekend at an American Society of Clinical Oncology conference in Chicago. Here are the highlights:
OVARIAN CANCER
French researchers found that it’s safe to avoid removing lymph nodes that appear healthy during surgery for advanced ovarian cancer. The study compared the results for 379 patients — half had their lymph nodes removed and half did not. After nine years, there was no difference in how long the patients lived and those with less-extreme surgery had fewer complications, such as the need for blood transfusions. The research was funded by the National Institute of Cancer in France.
ESOPHAGEAL CANCER
This German study looked at 438 people with a type of cancer of the esophagus that can be treated with surgery. Half received a common treatment plan that included chemotherapy and surgery on the esophagus, the tube that carries food from the throat to the stomach. Half got another approach that includes radiation too. Both techniques are considered standard. Which one patients get can depend on where they get treatment.
After three years, 57% of those who got chemo and surgery were alive, compared to 51% of those who got chemo, surgery and radiation. The German Research Foundation funded the study.
HODGKIN LYMPHOMA
A comparison of two chemotherapy regimens for advanced Hodgkin lymphoma found the less intensive treatment was more effective for the blood cancer and caused fewer side effects.
After four years, the less harsh chemo kept the disease in check in 94% of people, compared to 91% of those who had the more intense treatment. The trial included 1,482 people in nine countries — Germany, Austria, Switzerland, the Netherlands, Denmark, Sweden, Norway, Australia and New Zealand — and was funded by Takeda Oncology, the maker of one of the drugs used in the gentler chemo that was studied.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
]]>Following years of public outcry about the high cost of inhalers, the two drugmakers — along with a third, GlaxoSmithKline — have committed to capping the out-of-pocket cost at $35 a month. GSK’s cap is expected to take effect by Jan. 1.
The moves mirror similar steps taken by insulin manufacturers last year following the passage of the Inflation Reduction Act.
Like insulin, the cost of inhalers in the U.S. is significantly higher than in other wealthy countries. An investigation by the Democratic-led Senate Committee on Health, Education, Labor and Pensions noted AstraZeneca charges $645 in the U.S. for the same inhaler it charges $49 for in the U.K. Teva Pharmaceuticals, another major inhaler manufacturer, charges $286 in the U.S. for an inhaler that costs $9 in Germany.
Caycee Shapland, 29, from Omaha, Nebraska, spends at least $80 each month on her 4-year-old son Jackson’s Symbicort, an inhaler from AstraZeneca, to manage his asthma. He also takes albuterol, also from AstraZeneca, for his asthma.
Despite having health insurance, Shapland said the cost can balloon to $350 a month — a significant financial burden — depending on the severity of Jackson’s asthma.
“Going down from at least $80 a month to $35 a month is astronomical,” Shapland said. “I mean, feeding three young boys 5 and under is $300 a week on our groceries alone. So, it’s a lot of money.”
Dr. Alan Baptist, the division chief of allergy and immunology in the department of internal medicine at Henry Ford Health in Detroit, said the price caps for inhalers should provide significant financial relief for the 30% of his patients who can’t afford their medication.
“I applaud the companies for putting that out and I was surprised by it,” Baptist said.
High prices and lack of access to inhalers, he said, play a role in the racial disparities seen in asthma care, both in Detroit and nationwide.
While asthma rates are slightly higher in Black Americans than in white Americans, “when you look at the outcomes, the adverse events, it’s so much worse,” he said. Black children were 4.5 times more likely to be hospitalized for asthma than white children, and 7.6 times more likely to die from asthma, according to the federal Office of Minority Health.
‘Chaos for patients’
More than 27 million people in the U.S. have asthma, including 5 million children, according to the Asthma and Allergy Foundation of America.
Dr. Steven Stryk, an allergist-immunologist and associate professor of internal medicine at Oakland University William Beaumont School of Medicine, said that while the price caps are a big deal, he remains skeptical until he sees how they’re implemented.
“I think what you’ll find is most doctors saying I’ll believe it when it happens,” he said.
According to a spokesperson for AstraZeneca, both privately insured and uninsured patients will be eligible for the $35 price cap, which will apply to all of the inhalers the drugmaker sells in the U.S.
A spokesperson for Boehringer Ingelheim said the $35 cap will be automatically applied at the pharmacy counter for the majority of eligible patients with commercial insurance. For those without insurance or whose pharmacies aren’t participating, they’ll be able to visit the company’s website starting Saturday, where they can enroll in a copay card that will reduce the out-of-pocket cost to $35.
GSK’s price cap will go into effect later this year, a spokesperson said, and will be available to all patients, regardless of income.
People enrolled in government insurance programs, such as Medicare and Medicaid, won’t be eligible for any of the price caps due to federal restrictions.
Out-of-pocket costs for inhalers can vary widely, depending on the medication and insurance coverage, said Dr. Megan Conroy, a pulmonologist and critical care specialist at Ohio State University’s Wexner Medical Center. “It really creates a lot of chaos for patients.”
Kiowa Rix, 27, of Warren, Michigan, found herself with a $500 out-of-pocket price tag for her son Lucas’ inhaler — Flovent, from GSK — in February, when her insurance stopped covering the medication. The 6-year-old has severe asthma and needs to use an inhaler twice a day.
Lucas’ doctor switched him to a different inhaler, from Merck, which cost $80 a month. Rix is now switching him to Symbicort, which will be capped at $35.
“It makes me feel a little better that they’re realizing they’re overcharging,” Rix said of the price caps going into effect. “You shouldn’t have to go through all these hoops just to get something lowered that you or your child needs.”
While the price caps are significant, it’s unclear whether they’ll apply to the cost of all of a patient’s asthma drugs, or $35 per inhaler.
Patients, said Conroy of Ohio State, often require a rescue inhaler for quick relief, as well as a long-acting or maintenance inhaler to prevent symptoms.
“Patients have multiple medical comorbidities that they’re treating, and a longer list of medications beyond just inhalers for their respiratory disease, some of which carry similar stories of high copays,” she said.
Devastating consequences
The price caps should at least provide a sense of relief to families that qualify, said Dr. Ixsy Abigail Ramirez, a pediatric pulmonologist at University of Michigan Health. Some families, she said, have been forced to consider skipping, delaying or going without the medication because of the high cost, which can add up to thousands of dollars a month.
“Am I going to pay for food and the roof over my head this month? Or am I going to pay for an inhaler that my child requires to breathe so that we don’t end up in the hospital incurring other costs?” she said.
Cole Schmidtknecht typically spent around $5 for his inhaler. According to his father, Bil, one day when he tried to refill his prescription, he was told it would cost more than $500, which he couldn’t afford. Cole suffered a severe asthma attack days later, leading to a fatal cardiac arrest. He was 22.
“Had he had an affordable option in front of him, he’d have probably been here today,” said Bil.
Baptist, of Henry Ford Health, said that while the price caps are a step in the right direction, they don’t go far enough.
“In some ways, it’s just a Band-Aid on the bigger problem that we have,” Baptist said. “The real problem is the outrageous cost of pharmaceutical and drug prices in the United States.”
This story first appeared on NBCNews.com. More from NBC News:
]]>Lisa Pisano was the second person to receive a kidney from a gene-edited pig, and NYU Langone Health announced that she is stable after an operation to remove the organ earlier this week.
The first patient to receive a pig kidney transplant, Richard “Rick” Slayman at Massachusetts General Hospital, died in early May, nearly two months after his transplant. Doctors there said there was no indication he died as a result of the experimental transplant.
Pisano’s heart and kidneys were failing when, in a dramatic pair of surgeries in April, doctors implanted a mechanical pump to keep her heart beating and then the pig kidney.
At first she seemed to be recovering well. But Dr. Robert Montgomery, who led the transplant, said there were “unique challenges” to managing both the heart pump and new kidney. Her blood pressure dropped too low multiple times for optimal blood flow to the kidney.
The kidney lost function until doctors no longer could justify keeping her on immune-suppressing medications, Montgomery said in a statement Friday.
A recent kidney biopsy showed no signs of rejection – the biggest concern in highly experimental animal-to-human transplants – but there was “significant injury” from insufficient blood flow, he said. NYU will further study the explanted kidney for further insight on how it reacted inside a living person.
Montgomery noted Pisano wasn’t a candidate for the life-prolonging heart pump while on dialysis, and her heart disease in turn barred a traditional kidney transplant.
“We are hoping to get Lisa back home to her family soon,” he said. “Her strength and bravery in the face of adversity inspires and drives us as we continue pursuing the hope and promise of xenotransplantation.”
Pisano told the Associated Press in April that she knew the pig kidney might not work but “I just took a chance. And you know, worst case scenario, if it didn’t work for me, it might have worked for someone else.”
More than 100,000 people are on the U.S. transplant waiting list, most who need a kidney, and thousands die waiting. In hopes of filling the shortage of donated organs, several biotech companies are genetically modifying pigs so their organs are more humanlike, less likely to be destroyed by people’s immune system.
Formal studies of such organs are expected to begin next year. Meanwhile, NYU and other research teams have temporarily transplanted pig kidneys and hearts into brain-dead bodies, with promising results. In addition to the Mass General pig kidney transplant, the University of Maryland transplanted pig hearts into two men who were out of other options, and both died within months.
]]>The Food and Drug Administration posted its initial review of the drug Friday, ahead of a meeting of outside advisers who could help decide whether MDMA — currently illegal under federal law — becomes the first drug of its kind to win U.S. approval as a medication.
In their assessment, FDA scientists said that patients who received MDMA and talk therapy showed “rapid, clinically meaningful, durable improvements in their PTSD symptoms.” But they also called the research “challenging to interpret,” and questioned how long the benefits might last.
They said it’s difficult to know how much of the improvement came from MDMA versus simply undergoing intensive therapy, and also raised several safety concerns, including MDMA’s heart risks and potential for abuse.
The outside experts will take a nonbinding vote on the drug’s overall benefits and risks during Tuesday’s meeting. The FDA will make the final decision, likely in August.
Antidepressants are now the only FDA-approved drugs for post-traumatic stress disorder, which is closely linked to depression, anxiety and suicidal thinking and is more prevalent among women and veterans.
If approved, MDMA would be reclassified as a prescription medicine and made available to specially certified doctors and therapists. Currently, the drug is in the same ultra-restrictive category as heroin and other substances the federal government deems prone to abuse and devoid of any medical use.
MDMA, also known as ecstasy or molly, is the first in a series of psychedelics that are expected to be reviewed by the FDA in coming years. It’s part of a resurgence of research into the potential of psychedelics for hard-to-treat conditions like depression, addiction and anxiety. MDMA’s main effect is triggering feelings of intimacy, connection and euphoria.
Companies are studying MDMA, psilocybin, LSD and other mind-expanding drugs for numerous mental health problems.
Until recently, psychedelic research was mainly funded by a handful of nonprofit advocacy groups, including Multidisciplinary Association for Psychedelic Studies, or MAPS. The company seeking approval for MDMA, Lykos Therapeutics, is essentially a corporate spinoff of MAPS, which conducted all the studies submitted for FDA review.
In two studies, patients received MDMA as part of an intensive, four-month course of talk therapy lasting more than a dozen sessions, only three of which involved taking the drug. The drug is thought to help patients come to terms with their trauma and let go of disturbing thoughts and memories.
The approach was studied in nearly 195 adults with moderate-to-severe PTSD who were randomly assigned to undergo the therapy with MDMA or with a dummy pill. Following treatment, patients who received MDMA had significantly lower PTSD scores and were more likely to be in remission.
But FDA reviewers noted that the vast majority of patients correctly guessed whether they had received MDMA or a dummy pill, making it “nearly impossible” to maintain the so-called “blinded” objectivity considered essential for high-quality drug research. The agency also questioned how long the drug’s benefits might last. The studies tracked some patients for up to two years, but reviewers noted that about a quarter of patients quickly dropped out of the follow-up study, limiting the usefulness of the results.
The most common side effects of MDMA included headache, nausea, muscle tightness and decreased appetite. More serious issues included heart palpitations and elevated blood pressure, which FDA reviewers said had the “potential to trigger” life-threatening heart problems.
They also raised concerns about the potential for patients to abuse MDMA, which functions similarly to amphetamines and other stimulants.
While MDMA would be a first-of-a-kind approval, U.S. doctors and the FDA itself have already laid some of the groundwork for working with drugs that can cause intense, psychological experiences.
Hundreds of clinics across the U.S. already offer ketamine — the powerful anesthetic sometimes used as a party drug — to treat a host of ailments, including depression, anxiety, chronic pain and PTSD. The FDA has only formally approved the drug for use during surgery, but its availability allows doctors to prescribe it “off-label” for various mental and physical ailments.
In 2019, the FDA approved Johnson & Johnson’s proprietary form of the drug, Spravato, a nasal spray that treats severe depression. Similar to ketamine, the drug is offered at doctor’s offices and clinics where patients usually spend several hours reclining in a chair.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
]]>So-called ghost poops have gone viral on TikTok, where you can find countless videos of people talking about the mysterious fecal phenomenon.
Typically, when we go No. 2, we see some evidence in the toilet bowl or on toilet paper. When you could’ve sworn you passed stool but there’s no sign of it, you may have had a “ghost poop.”
While “ghost poop” isn’t a term you’ll find in the medical literature, “patients definitely are interested in talking about ghost poops and ask me about this commonly,” Dr. Felice Schnoll-Sussman, gastroenterologist and professor of clinical medicine at Weill Cornell Medicine, tells TODAY.com.
What is a ghost poop?
According to social media and gastroenterologists, a ghost poop can refer to a few different bowel-related phenomena:
- The sensation of needing to poop, which ends up being gas
- A stool that sinks to the bottom of the toilet and disappears
- A stool that leaves no trace on toilet paper after wiping
In most cases, pooping is a well-orchestrated and coordinated movement, Dr. Rabia De Latour, gastroenterologist at NYU Langone Health, tells TODAY.com. The nerves in our rectum, our brain and the anal sphincter muscles all work together to release stool at the right time.
“The sphincters in our rectum are incredibly intelligent and sensitive parts of our body,” says De Latour. This part of the body can actually distinguish between air, liquid and solid and selectively let one thing out and not the other, she adds.
During defecation, “the rectum senses pressure from stool that has entered it and lets your body (and brain) know it’s time to defecate,” says De Latour. The anal sphincter muscles then push the stool out of the anus in a controlled way, says De Latour.
Sometimes, the bowel movement we sense coming isn’t poop at all — it’s gas. You may feel the sensation of needing to go No. 2, sit on the toilet, and try to push but nothing comes out, says De Latour. During this type of phantom poop, your body and brain gear up for a bowel movement without actually having one.
Another type of ghost poop is one that passes quickly and sinks to the bottom of the toilet and disappears before you can see it. You may go to flush and realize there’s no sign of stool in the bowl.
“It is so well-formed and you pass it so easily that you barely even know,” says Schnoll-Sussman. All stool will eventually sink, she adds, but some are heavier and sink immediately.
The final type of ghost poop, sometimes called a ghost wipe, is poop that leaves no visible residue on toilet paper after wiping, or no trace after washing — no matter your preferred post-poop hygiene method, you can’t find any evidence afterwards.
What causes ghost poops?
There are several explanations for why you might experience each type of ghost poop, according to the experts.
The body can normally distinguish between stool and gas and which it’s letting out, De Latour notes. However, sometimes too much gas can build up in the rectum and feel like stool or tension, which may give your brain the cue to sit on the toilet.
The rectum feels full, “and the sphincters get stimulated, and you do pass something, but it’s just air,” says Schnoll-Sussman.
This can occur after eating too many gas-causing foods, says De Latour, or simply from being bloated. Excess gas in the intestines can also be caused by constipation, digestive disorders, bacterial imbalances and food intolerances, the experts note.
Looking at the second definition of ghost poops, whether a stool floats or sinks has a lot more to do with what we eat, says Schnoll-Sussman. “When we have diets that are higher in fiber, they can make the bowel movement more formed, so it can go straight down the toilet,” she adds. Non-absorbable or undigested food such as seeds or corn can also make the stool heavier.
“That’s perfectly normal. … Waste is supposed to be dense,” says De Latour.
Stool that floats often contains more fat or gas, says Schnoll-Sussman. This may occur if someone has a high fat diet or their body can’t absorb fat appropriately, she adds. Gassy, floating stool can also result from excess gas in the digestive tract.
Sometimes, whether a stool sinks and disappears is more about the aim and the architecture of the toilet, the experts say.
When wiping after a poop, it’s normal for some remnants of a bowel movement to remain in or on the anus, the experts note, which can usually be removed with a few wipes with toilet paper or a quick wash. But when a bowel movement leaves no residue or trace after wiping, this generally just means the stool is very well-formed, firm, and easily passed, the experts note.
The texture of stool and how much you need to wipe can depend on a few factors: fiber intake, gut health, the amount of water and other nutrients absorbed from stool in the large intestine, and how well the anal sphincter muscles function, Schnoll-Sussman explains.
Are ghost poops healthy?
A stool that is easily passed, disappears to the bottom of the toilet, and leaves no trace after wiping, is nothing to worry about. In fact, it is probably a good sign, the experts note.
“If (someone) has a bowel movement and it’s so clean and well-formed that it doesn’t leave any residue and just sinks, that is actually a very healthy bowel movement,” says De Latour.
But if you regularly feel like you need to go No. 2 but aren’t able to go or are only passing gas, it may be time to see a doctor. While it could be something as simple as excess gas in the digestive tract, these types of phantom poops could be a sign of a health problem.
Tenesmus is the urge to pass a bowel movement without being able to defecate, says De Latour. “You still feel like there’s something in there, but nothing is coming out of the rectal vault,” De Latour adds.
This often results from inflammation in the rectum, which can mess up the signaling between the nerves and our brain, De Latour adds. Potential causes include inflammatory bowel disease, Crohn’s, colorectal polyps, and anal cancer, per the Cleveland Clinic.
In most cases, a ghost poop is nothing to be spooked by — but always talk to your doctor if you have questions or concerns, or notice sudden changes in your bowel habits.
Other poop problems to look out for
Health care professionals use a tool called the the Bristol Stool Chart to classify poop into seven different types based on shape and consistency, per the Cleveland Clinic.
“You want it to be in the middle of that chart (type 3 or 4), a nice sausage-shaped firm stool,” says De Latour.
Abnormal shape or consistency
Types 1 and 2 usually are a sign of constipation. Stool should not be too hard, which can cause straining. Continually having type 5 poops could be a sign of bowel issues due to lack of fiber, and types 6 and 7, diarrehea, usually indicate an illness or other digestive issue.
Excess wiping
If you find yourself wiping endlessly after going No. 2, this could be due to excess tissue in the rectum, which can be caused by severe inflammation, hemorrhoids, anal skin tags and other conditions, says Schnoll-Sussman.
“These can make it a bit more challenging (to defecate), and allow the remnants of the bowel movement to become stuck and need to be wiped off,” Schnoll-Sussman adds.
Persistent loose stool
Loose stools, also known as diarrhea, also tend to leave more of a mess, De Latour explains. It’s normal for most people to pass loose stools from time to time, but severe or persistent loose stools could be a sign of viral or bacterial infection, digestive disorders, and other health problems.
Reduced control over bowel movements
Pelvic floor dysfunction, rectal nerve issues, and weakened anal sphincter muscles can also impact continence, or our control over bowel movements, and how much stool or residue is left behind, the experts note.
Blood or pain
No matter how much you have to wipe, never ignore blood or pain, says De Latour.
This article first appeared on TODAY.com. Read more from TODAY here:
]]>Nearly a dozen scientists from the university’s Center for Neural Engineering and Prostheses have worked for several years to design a decoding system that could turn the man’s brain activity into sentences in both languages and display them on a screen.
An article published May 20 in Nature Biomedical Engineering outlining their research identifies the man as Pancho. At age 20, he became severely paralyzed as a result of a stroke he had in the early 2000s. Pancho can moan and grunt but can’t articulate clear words. He is a native Spanish speaker who learned English as an adult.
Under the leadership of Dr. Edward Chang, a neurosurgeon who serves as co-director of the Center for Neural Engineering and Prostheses, Pancho received a neural implant in February 2019, allowing scientists to start tracking his brain activity.
By using an AI method known as a neural network, researchers were able to train Pancho’s implant to decode words based on the brain activity produced when he attempted to articulate them. This AI training method basically allows the brain implant, known scientifically as a brain-computer interface device, to process data in a way that is somewhat similar to the human brain.
By 2021, the technology had significantly helped restore Pancho’s ability to communicate, but only in English.
“Speech decoding has primarily been shown for monolinguals but half the world is bilingual with each language contributing to a person’s personality and worldview,” Chang’s research group said on X. “There is a need to develop decoders that let bilinguals communicate with both languages.”
However, the 2021 research served as the foundation to develop the decoding system that later made Pancho’s brain implant bilingual in Spanish and English.
Allowing a language switch based on preference
After discovering that Pancho’s brain had “cortical activity” across both languages years after he became paralyzed, the scientists realized they could leverage that to train a bilingual brain implant without the need to train separate language-specific decoding systems.
“We leveraged this finding to demonstrate transfer learning across languages. Data collected in a first language could significantly expedite training a decoder in the second language,” Chang’s research group said on X, because it is based on the brain activity produced by “the intended vocal-tract movements of the participant, irrespective of the language.”
In 2022, the scientists sought out to prove that. They again used the artificial neural network to train Pancho’s brain implant on the distinct neural activity produced by his bilingual speech.
According to their findings, Pancho was able to use the bilingual decoding system powering his brain implant to “participate in a conversation, switching between [both] languages on the basis of preference.”
The study ultimately shows “the feasibility of a bilingual speech neuroprosthesis,” or bilingual brain implant, and provides a glimpse into how this type of technology has the “potential to restore more natural communication” among bilingual speakers with paralysis, according to the May 20 article.
This story first appeared on NBCNews.com. More from NBC News:
]]>“I thought it was sun poisoning,” she said.
She woke that night in a sweat and spent the hours alternately burning up then freezing. In the morning, her eyes were sore and her lymph nodes were swollen. For the following week, there was nothing to do but sleep, stay hydrated and wait for the body aches that give the illness the moniker “break-bone fever” to pass.
Latin America is experiencing its worst dengue fever outbreak on record. Case numbers in the first 4 ½ months of 2024 are already 238% higher than they were by this time last year, which itself ended with a record 4.1 million cases, according to the Pan American Health Organization. Cases are more than 400% higher than the five-year average.
An unusually wet and warm summer season brought by the El Niño weather pattern has created ideal conditions for the mosquitoes that spread dengue to hatch en masse and carry higher amounts of the virus.
Experts warn this could be a preview of what dengue fever will look like in the future. Climate change is creating unusually balmy conditions, which are already expanding the range of mosquito-borne diseases.
“That’s concerning for places where dengue hasn’t occurred before in recent history: North America and Europe,” said Dr. Albert Ko, a professor of epidemiology of microbial diseases at the Yale School of Public Health.
Dengue is a viral fever caused by four different viruses and spread through mosquito bites. It’s common in many tropical regions across the globe, but has begun to appear in more temperate climates. The mosquitoes that carry dengue fever, Aedes aegypti are now regularly found in the southern parts of the U.S., but recently, the insects have been found as far north as the Bay Area and Washington, D.C. One 2019 study predicted an additional 2 billion people will be at risk for dengue fever by 2080.
“We are definitely worried,” Ko said.
Why are dengue cases rising around the world?
Dengue outbreaks have historically occurred in the Americas every three to four years, said Dr. Gabriela Paz-Bailey, dengue branch chief in the division of vector-borne diseases at the Centers for Disease Control and Prevention. “But now we are seeing them every year,” she said.
Part of the reason for that is tied to climate change.
A warming climate expands the mosquitoes’ habitat and allows them to breed all year long, rather than only in the warmer months. The hotter temperatures also cause the viruses to replicate faster, meaning mosquitoes end up carrying many more viral copies, increasing the likelihood that a person will become infected if bitten.
“We are also seeing dengue cause outbreaks at times when they usually don’t occur,” Ko said.
South America’s dengue cases weren’t just unusually high this year, but they also came unusually early in the season. Similarly, Puerto Rico, a place where dengue outbreaks can occur in the summer and fall, declared a public health emergency in late March after the U.S. territory was overtaken by dengue fever cases and more than 400 people were hospitalized.
In recent years, the epidemic has spread to parts of southern Brazil and northern Argentina, where dengue hasn’t previously been a big problem, Ko said.
“That gives us a snapshot of what we may see here in North America in the coming decades,” Ko said.
How would dengue get a foothold in the U.S.?
The fact that Aedes aegypti mosquitoes are found in places outside their normal range doesn’t mean the mosquitoes are carrying dengue viruses, but those first insects are a warning of what may be to come, Ko added
Locally transmitted dengue fever infections — meaning the infected person didn’t get sick abroad — are still rare in the continental U.S., but have recently been seen for the first time in some states. Last October, California health officials reported the state’s first case of locally transmitted dengue in Pasadena. Local transmission has also occurred in Arizona, Florida and the southern coast of Texas. Last summer brought record-breaking heat waves to Europe, where cases of local dengue transmission were seen in France, Italy and Spain.
“I think this means dengue will become more common,” said Paz-Bailey, adding that the main concern is still the significant increase in cases where the virus is already endemic.
This summer, she does not expect to see significant dengue outbreaks on the U.S. mainland, but she said there is likely to be some people who travel to regions that have higher-than-usual cases and bring the virus back home.
“Travel-associated cases do result in small chains of outbreaks,” Paz-Bailey said.
Humans are reservoirs for dengue, so in order to have widespread transmission, enough people must be infected for the mosquitoes to reliably bite someone with the virus so that they can spread it to another person.
“That’s why we’re seeing an outbreak of dengue in Puerto Rico right now,” said Michael von Fricken, director of the One Health Center of Excellence at the University of Florida in Gainesville. “They’ve reached this tipping point where there are enough infected humans that they’re subsequently infecting other mosquitoes that are continuing to transmit disease.”
Florida has logged 176 dengue cases so far this year, the vast majority in people who were infected in other countries, most frequently Brazil or Cuba. The Florida Health Department has recorded only seven cases of locally transmitted dengue transmission in the state so far this year. In all of 2023, the department documented 173 locally transmitted cases, most of them in Miami-Dade County.
What are the symptoms of dengue fever?
Dengue fever is caused by four viruses, so a person can be infected four times in their lifetime. Only about 1 in 4 people are symptomatic the first time they’re infected, according to the CDC.
Ko said a person’s initial symptoms are usually a fever and headaches. Fatigue, nausea, vomiting, a rash that looks like measles, as well as the extremely painful body aches.
Most people recover in a week or two, but about 1 in 20 people develop severe dengue, which can be fatal. The more times a person is infected with dengue, the higher risk they are for complications.
“After you’ve had your first exposure, your risk of having dengue hemorrhagic fever or severe symptoms increases exponentially,” Von Fricken said. Dengue also becomes deadlier with each infection.
While the U.S. does have a dengue vaccine, it’s approved only for children ages 9 to 16 who live in places where dengue is endemic, including Puerto Rico, American Samoa or the U.S. Virgin Islands.
What’s more, children can get the vaccine only if they’ve previously had a dengue infection. That’s because if a person were to get vaccinated and then get their first dengue infection, they still run the risk of getting very sick, just as someone gets sicker from their second infection. Since most Americans have not had dengue, “that vaccine is not very useful” for most, Ko said.
There’s no specific drug to treat dengue. Instead, doctors just aim to treat the symptoms and keep the patient comfortable until the virus runs its course. That means resting and drinking a lot of fluids. Ko said people should try to take acetaminophen (Tylenol) for pain and fever if they can, since nonsteroidal anti-inflammatory drugs (NSAIDs), which include ibuprofen and aspirin, can make bleeding worse if someone develops hemorrhagic dengue, in which their blood vessels are damaged and become leaky.
Paz-Bailey said it’s important for people traveling to places with dengue to stay in places with air conditioning when possible, use insect-repellant and wear long sleeves and pants to avoid mosquito bites.
Bed nets can be helpful, but the mosquitoes that carry dengue typically bite during the day, so they may be less helpful than they are at preventing other mosquito-borne diseases like malaria, Ko said.
At home, people can make their yards less appealing to mosquitos by reducing the amount of standing water, especially after a bout of rain.
“It’s difficult to control the mosquito population, so we need to hit it with all we have and layer our strategies,” Paz-Bailey said. “No single strategy will be good enough.”
This story first appeared on NBCNews.com. More from NBC News:
]]>The most common type of dementia, Alzheimer’s disease, affects nearly 6 million Americans and is expected to rise to 14 million by 2060 due to our aging population. Cognitive decline — an impairment of memory, decision-making and ability to learn — develops due to aging neurons and the slowing down of the speed at which the brain functions. It’s directly linked to the aging process and leads to worsening memory, attention and brain processing.
Beyond the calories that are burned by running all the many functions of the brain, there are specific foods that help support our brain’s activity. Here’s what to know about so-called brain foods.
New research on foods and brain aging
A new study published in the journal Nature Aging points to specific nutrients that can contribute to slower aging in the brain. The 100 participants between 65 and 75 years old completed questionnaires, underwent various physical and cognitive tests, MRI scans and had their blood plasma drawn after fasting.
Researchers found that one group had signs of slower aging and also ate a specific nutrient profile. The nutrients in the blood that were prevalent in participants with slower aging were:
- Fatty acids, found in seafood and some healthy cooking oils
- Antioxidants, found in berries, garlic, tomatoes, nuts and plenty of other fruits and vegetables
- Carotenoids, found in spinach, kale, carrots, broccoli and some fruits
- Vitamin E, found in fruits, vegetables, seafood, seeds and nuts and more
- Choline, found in egg yolks, beef, dairy and some veggies
Many foods that make up the Mediterranean diet are high in these nutrients, the researchers noted. While most previous research on foods and brain health have relied on food questionnaires, this research is one of the first to use blood biomarkers, brain scans and cognitive testing.
What is the No. 1 best food for brain health?
As a registered dietitian, I would say this is the best food to boost your brain health:
Fatty fish
Studies have shown that eating just one seafood meal per week has been linked with a lowered risk of both Alzheimer’s and dementia. Our brains are mostly made up of omega-3 fatty acids called EPA and DHA, so it makes sense that foods that contain these fats would help support brain health.
Omega-3 has been shown to help protect the brain with its anti-inflammatory effects, ability to help create new neurons, and power to help clear the brain of plaques, one of the signs of Alzheimer’s. The best-known sources of EPA and DHA on the planet are high-quality seafood, like wild Alaskan salmon, sablefish and halibut. Sardines are another source of omega-3s. Wild-caught seafood is sustainably caught and also has lower contaminants than farm-raised seafood.
What foods help with brain health?
Eggs
The micronutrient choline is finally getting the attention it deserves for its role in brain health, including memory, thinking, mood and more. Higher levels of choline intake are thought to support brain function, which may decrease the risk of some types of dementia, including Alzheimer’s. One of the best dietary sources of choline is the egg. One large egg provides 150 milligrams, about 25% of the daily requirement for men and 35% for women.
You’ll find choline (plus nearly half of an egg’s protein and many other vitamins and minerals) in the yolk, so be sure to eat the whole egg. According to the American Heart Association, eggs can be included as part of a heart-smart diet for healthy adults.
Walnuts
Research has found that eating walnuts may be linked with improved cognitive function and memory in groups at high risk for age-related cognitive impairment, and reduced risk of Alzheimer’s disease. The nut is also linked with a reduced risk of other diseases, such as cardiovascular disease or Type 2 diabetes, which are both risk factors for developing dementia. Whether you’re munching on walnuts for heart or brain health, you can feel good knowing that you’re covering both bases.
Berries
Known for being rich in antioxidants and polyphenols, berries contain several disease-fighting compounds. Research has found that eating berries has a protective effect against cardiovascular disease, cancer and Alzheimer’s. A major contributor to Alzheimer’s and other chronic diseases is inflammation. Both strawberries and blueberries have anti-inflammatory benefits.
A study on strawberries found that when older adults, ages 60 to 75, were given the equivalent of 2 cups of strawberries daily for 90 days, they showed improvement in memory and learning tests. In a similar study, participants who ate the equivalent of 1 cup of blueberries daily were tested on verbal learning and task switching and had significantly fewer errors on both tests at 45 and 90 days.
Prunes
Known for their gut health and bone benefits, prunes are also great for your brain. Prunes are high in potassium and a source of vitamin B6 and copper, all micronutrients that contribute to normal functioning of the nervous system. What’s more, studies on prunes show that the dried fruit has anti-inflammatory, antioxidant and memory-improving characteristics. The benefits are likely due to the high content of anthocyanin, a blue plant pigment.
Citrus fruits
One of the markers of Alzheimer’sdDisease is neurodegeneration. The peel of a small citrus fruit from Okinawa, Japan called shikuwasa lime (also called citrus depressa) is rich in a plant compound called nobiletin. Nobiletin has been found to protect nerve cells and provide anti-inflammatory benefits and is looking promising as a potential treatment for Alzheimer’s. The good news is that this important compound can also be found in mandarins, oranges, tangerines and grapefruits.
Cocoa powder and dark chocolate
Cocoa beans are rich in flavanols, which help fight inflammation in our body and can increase blood flow to the brain. Choosing dark chocolate over milk chocolate helps you get more of the protective polyphenols.
Extra virgin olive oil
As the staple of the Mediterranean diet, extra virgin olive oil is rich in polyphenols and vitamin E. A 2023 study done at the Harvard T.H. Chan School of Public Health found that daily consumption of more than half a tablespoon of EVOO had a 28% lower risk of dying from dementia compared to never or rarely consuming olive oil. The study also found that replacing just one teaspoon of margarine or mayo with the same amount of EVOO daily was associated with an 8 to 14% lower risk of dying from dementia.
Tips to sharpen your memory
In addition to what we eat, there are other habits to work on to support brain health, Dr. Andrew Budson, author of “Why We Forget and How to Remember Better,” tells TODAY.com.
Here are some strategies to remember things better:
- Focus your attention on whatever it is that you want to remember.
- Organize whatever it is that you want to remember, whether it is by reviewing the sights, sounds, smells, thoughts, and feelings of an experience or the material you need to memorize for a presentation or exam.
- Understand what you want to remember, such as the deeper meaning or implications of an episode of your life or the individual elements of your presentation or exam
- Relate what you are learning to things you already know or have experienced
In addition to the tips above, you may want to ditch some habits that can hinder memory over time, Budson says. These include:
- Not correcting bad habits immediately. Break bad habits right away or they will become part of your routine. For example, don’t leave your keys, wallet, cell phone where they are difficult to find— even once —or you may find yourself frequently hunting around the house looking for them.
- Not paying attention to where you are or what you are doing. This is the No. 1 reason people have trouble finding their car, keys, phone, etc. Stop and pay attention to where you parked and where you put down your keys, for example.
- Not engaging in aerobic exercise regularly. Aerobic exercise releases growth factors from the brain that help to grow new brain cells in the hippocampus, the part of the brain that forms new memories.
- Being sedentary and watching too much television. There are new studies that suggest that even when controlling for vigorous exercise, it is still important to not be sedentary and not to watch more than one hour of television per day.
- Eating too much unhealthy food. Everyone can get away with eating dessert, red meat, butter, soda, refined sugar and flour once in a while, but it is important that the majority of one’s diet be from the Mediterranean menu, including fish, olive oil, fruits, and vegetables, nuts and beans, and whole grains.
- While Budson doesn’t recommend any particular supplements for brain health, he does encourage people to have their vitamin D and B12 levels checked by your physician at least once every decade after age 40. Both vitamin D and B-12 are necessary for proper memory function.
This story first appeared on TODAY.com. More from TODAY:
]]>Health officials are racing to get the world to agree to new ways to prepare for and fight an inevitable future pandemic. COVID-19 is fading into history as elections and crises like climate change and war compete for the public’s attention.
A bold project to adopt a pandemic “treaty” at this week’s World Health Assembly was shelved on Friday as 2 1/2 years of work ran into disagreements over sharing information about pathogens that cause pandemics and the technology used to fight them.
Experts say the best chance now to address pandemics at the assembly will be proposed changes to the WHO’s International Health Regulations, which were set up in 2004. Amendments would urge countries to boost alert, detection and containment capacities and cooperate internationally.
One proposal would let the WHO director-general declare a “pandemic emergency.”
Envoys say a deal is close, but similar disagreements between rich countries and developing ones that set back the pandemic treaty negotiations linger. Issues remain over proposed “transfer of technology” and the creation of a new fund under WHO in 2030 that would help boost pandemic-fighting capacities “particularly in developing countries.”
WHO Director-General Tedros Adhanom Ghebreyesus insists the stalled work on the pandemic treaty was not a failure, and acknowledged an “immense” task on a “very ambitious timeline” — alluding to the many years it usually takes for U.N. member countries to reach global treaties.
“Of course, we all wish that we had been able to reach a consensus on the agreement in time for this health assembly and cross the finish line,” Tedros said in opening remarks. “But I remain confident that you still will — because where there is a will, there is a way.”
“It’s now for this World Health Assembly to decide what that way is — meaning the solution is in your hands,” he added.
The premise is that pathogens that have no regard for national borders require a united response from all countries. But decision-makers have struggled to balance national interest with the call from WHO officials to think more broadly in the interest of humanity.
Health ministers now have to try to overcome deep-set differences, including how the world can share information on emerging pathogens and scarce resources like vaccines when demand skyrockets.
“If nothing comes out of WHA (the assembly), it’s a huge missed opportunity,” said Yuanqiong Hu, a senior legal and policy adviser at Doctors Without Borders, “If they don’t come up with a clear road map, how are they going to finish this process?”
]]>Smartphones and laptops help us to stay connected to our loved ones through phone calls and video conferencing, Laurie Santos, a psychology professor who teaches Yale’s most popular class ever, said in a recent podcast episode of “The Happiness Lab.”
At the same time, “research shows that our screens and apps and devices are making us less social, less present and even less happy,” Santos added.
During the episode, Santos spoke to Amy Blankson, a happiness expert and co-founder of the Digital Wellness Institute, about ways that people can achieve a better balance when using digital devices.
Here are a few of the tips that Blankson shared.
7 tips for developing ‘digital balance’ from happiness experts
“Digital balance is really finding that sweet spot. We call it a spot of ‘digital flourishing’ where it’s not that you are addicted to technology and it’s not that you’re swearing it off either,” Blankson said during the episode.
“It’s really that you’re finding that happy medium where technology is working for you, not the other way around.”
These are some of the tips that Blankson suggests for achieving digital balance:
- Practice the “really” rule: When you feel an urge to use your phone or turn on the TV, think about if it’s really necessary for you to do it in the moment or if it’s getting in the way of something else that you need to do. Ask yourself, “Do I really need to be using my device right now? Is this the best use of my time really?”
- Be mindful of the bottomless bowl: Some apps don’t have a limit to how long you can scroll on them, which is commonly referred to as the bottomless bowl, Blankson said. “Those are the apps that are the most dangerous for us, the ones that suck us in the most and the ones that require us as individuals to be stronger and more intentional about setting boundaries for ourselves,” Blankson added.
- Use stopping cues: To limit your time spent on devices, especially when using apps with the bottomless bowl structure, come up with a signal that will let you know how long you’ve been looking at your devices. Then use that signal as a sign that it’s time to take a break from using the device, Blankson said. A simple tool you can use as a signal is a timer.
- Switch your phone into grayscale mode when you want to put it down: In your phone’s settings, put your phone on grayscale mode. “Essentially what it does is it takes all of the color off of your screen, all the flashing lights, and sometimes you can turn off all the sound. So you get this very blank canvas,” Blankson said. “It’s so boring, you won’t want to look at it anymore.”
- Place your phone out of your line of sight: Put your phone behind your laptop or store it in a bag, so you’re unable to see it while you’re working on something. “The reason why this works is because the need to be needed is so strong that our eyes are actually flickering back and forth between our screen and our task or the person we’re with because we might be needed,” Blankson said. “By having it out of our line of sight, there’s nothing to go check. There’s nothing to interrupt us.”
- Engage in some screen-free activities: Plan to connect with others through screen-free activities. Some choices that Blankson recommends include playing board games, creating art, sitting in stillness, cooking and trying new foods. Make a list of screen-free activities you like and participate in one when you feel like reaching for your phone.
- Use technology to enhance your productivity: Consider using an app for journaling that reminds you to write about your day or think about a few things that you’re grateful for. Send a thoughtful text message once a day to one of your loved ones. Or try a meditation app to incorporate mindfulness into your day.
Want to be a successful, confident communicator? Take CNBC’s new online course Become an Effective Communicator: Master Public Speaking. We’ll teach you how to speak clearly and confidently, calm your nerves, what to say and not say, and body language techniques to make a great first impression. Sign up today and use code EARLYBIRD for an introductory discount of 30% off through July 10, 2024.
Plus, sign up for CNBC Make It’s newsletter to get tips and tricks for success at work, with money and in life.
]]>The medical condition — characterized by extreme sadness, anxiety or despair following childbirth — affects up to 1 in 5 women.
At five months postpartum, the woman was stuck in a haze, said Dr. Misty Richards, medical director of perinatal psychiatry at the Maternal Outpatient Mental Health Services Clinic at UCLA Health, who treated the patient and described her experience to NBC News.
“She wasn’t taking showers. She wasn’t eating,” Richards said, adding that the woman’s husband had taken a leave of absence from work to care for his wife and new baby.
Richards’ clinic has treated hundreds of these patients. At first, she connected the woman to an intensive outpatient program, but even while attending, the patient was actively suicidal, Richards said.
That’s when Richards prescribed zuranolone — the first-ever pill to treat postpartum depression.
The Food and Drug Administration approved the drug in August, but it took months for supply to become available. Several psychiatrists said they’re just starting to write their first prescriptions, since it has taken time to find good candidates for the drug who are willing to take it. They hope it will be a game changer because it’s fast-acting and can be taken at home.
Richards said the woman who took zuranolone, the first of her patients so far, saw her depression symptoms start to resolve around three days in. The patient was seeing dramatic results as of day eight and didn’t experience any side effects.
“She tells me she feels like she just woke up,” Richards said, adding: “I truly feel like I’m meeting her for the first time. Her husband was in tears, super grateful. Just a major, grand slam success story — which, by the way, we don’t tend to see in psychiatry.”
Postpartum depression can have severe consequences for mothers and their children. For moms, it may increase the risk of suicide, high blood pressure, diabetes or stroke. Mental illness, suicide and drug overdoses are the leading causes of death in the first year after a woman gives birth. Children born to mothers with postpartum depression, meanwhile, are more likely to have developmental delays and emotional or behavioral problems, and have a greater risk of dying before one year.
Before zuranolone, the only available treatment was an intravenous injection approved in 2019. But it comes with a risk of excessive sedation and sudden loss of consciousness, so only certain treatment centers are allowed to administer it and patients must stay in the hospital for 2 1/2 days. Other women with the condition are given standard antidepressants, but those usually take weeks to start working. (Zuranolone can be taken alongside widely used antidepressants).
The FDA fast-tracked zuranolone in 2017 — a step taken for drugs that could treat serious conditions and fill an unmet medical need. In a pair of clinical trials, it was shown to improve symptoms of severe postpartum depression — such as anxiety, difficulty sleeping, loss of pleasure, low energy, guilt or social withdrawal — as early as three days in. The pills are taken daily for two weeks, in the evening with a fatty meal.
The medication isn’t ideal for mild postpartum depression, or the “baby blues,” doctors said. Instead, they’re considering it for patients who have a hard time caring for themselves or their babies — in other words, those for whom medical intervention could be lifesaving.
Challenges in prescribing the new pill
Despite the potential benefits of zurnalone, psychiatrists said some patients are hesitant to take a drug that’s new to the market, wary of side effects or concerned about practical barriers.
Zuranolone can cause drowsiness, dizziness, diarrhea, fatigue and urinary tract infections. So far, doctors said they’ve heard of patients experiencing drowsiness or dizziness, but not to an extreme degree.
Because of this effect, however, the medication comes with a warning not to drive or operate heavy machinery for at least 12 hours after taking it.
Dr. Uruj Haider, medical director of consultation services at the Massachusetts Child Psychiatry Access Program for Moms, said some patients have expressed concern that they’ll be too tired to feed their babies at night. She recommends that patients have another caretaker in the house overnight.
“If they have toddlers and they don’t have someone at home to watch the baby or other children at night, that can be very challenging to be on a medication that can make you feel very drowsy,” she said.
Other patients have declined the medication due to a lack of safety data on breastfeeding, Haider added. A small amount of zuranolone can pass from mother to child through breast milk, but studies haven’t evaluated if it poses any harm.
Richards said she recommends that new moms discard their breast milk while taking zuranolone.
But Dr. Julia Frew, a psychiatrist at Dartmouth Hitchcock Medical Center, said she suspects that the benefits of breastfeeding outweigh the risk of medication exposure, particularly since the transfer of other antidepressants through breast milk hasn’t been shown to pose a significant risk.
“I think it could be a very reasonable choice for someone to continue breastfeeding while they’re taking it,” she said. “Some people may be uncomfortable with that, and they may want to choose to pump and dump.”
Additionally, zuranolone is classified as a Schedule IV controlled substance — in the same class as Xanax — meaning there’s a low risk of addiction.
“I don’t think we really know the addictive potential. There’s hope that it’s not addictive,” said Dr. Katrina Furey, a clinical instructor in the Yale School of Medicine’s psychiatry department.
Patients report improvements
Haider said one of her patients has completed a 14-day course of zuranolone, and the woman’s symptoms started to improve by day four.
“The only side effect was the drowsiness at night, and that was gone by the morning,” she said. The woman welcomed the drowsiness, Haider added, since it helped her sleep.
Frew similarly said she has had one patient finish zuranolone this year. The woman had chronic depression before her pregnancy, which got significantly worse postpartum. Other medications had failed to treat her symptoms, but zuranolone offered some relief, she said.
But it’s not yet known whether zuranolone has a lasting effect. In trials, patients saw a benefit for up to four weeks, but the studies didn’t follow people for longer than that.
“We don’t know yet if people are going to need booster doses down the road,” Furey said.
A ‘cumbersome, clunky’ insurance process
Some psychiatrists said they’ve struggled with the process of getting zuranolone approved by insurance companies.
The medication must go through one of five specialty pharmacies and be delivered to patients by mail.
“You cannot pick up zuranolone from your local CVS,” Haider said.
Insurance companies also have varying requirements about how severe patients’ symptoms need to be to get zuranolone covered. Some insurers require people to have tried and failed a standard antidepressant first.
“It’s a cumbersome, clunky process,” Richards said, adding that many patients don’t have time to wait for the kinks to iron out or actively manage their deliveries.
“If someone is severely depressed — and that is the reason you would be prescribing zuranolone instead of anything else — asking them to kind of wait for this process and then to engage in this process, it is hard,” she added.
Nevertheless, psychiatrists said they’re eager to recommend the drug to patients.
“I’ve started telling all my patients about it,” Furey said. “Just so they know it’s available and they know that there is this new option.”
This story first appeared on NBCNews.com. More from NBC News:
]]>Americans under the age of 30 reported lower levels of happiness from 2021 to 2023 than those over the age of 60, according to this year’s World Happiness Report.
Jonathan Haidt, a social psychologist at New York University’s Stern School of Business, lays the blame squarely on our devices.
His new book, “The Anxious Generation: How the Great Rewiring of Childhood is Causing an Epidemic of Mental Illness,” argues that the constant access to social media that phones have given us has led to social comparison, sleep deprivation and loneliness in Gen Z.
And it’s touched a nerve: his book is currently No. 3 on the New York Times nonfiction bestseller list.
Of course, as high-profile as the book has become, not everyone agrees with its thesis. Some critics argue blaming smartphones is an oversimplification and not fully supported by evidence
Zach Rausch, lead researcher to Haidt and an associate research scientist at NYU-Stern School of Business, says kids who had access to social media and iPhones in elementary and middle school are more anxious and less productive.
“The goal of technology is that it’s a tool that we use to meet our goals,” he says. “If it’s not doing that, it ends up using us at the cost of our goals.”
But, there are ways to curb these negative effects. Here are three things you can do today to increase your happiness and stay focused.
1. Buy an alarm clock.
Your phone being the last thing you interact with before bed and the first when you wake up can adversely affect your sleep and add to stress levels.
Purchasing an alarm clock and keeping your device outside your bedroom can create some physical and mental distance from social media.
2. Use your phone to meet with people in person.
Oftentimes, conversations on Instagram or text don’t cross over from digital to physical.
“We used flip phones to connect with each other in order to eventually meet in person,” Rausch says. “The online world is kind of the opposite. We connect in order to stay there. And our argument is that that’s not sufficient.”
Yale University happiness professor Laurie Santos echoes this sentiment.
“Every available study of happy people suggests that happy people are more social, they spend more time physically around other people, and they invest time in their friends and family members,” Santos, who teaches “The Science of Wellbeing” course at Yale told CNBC Make It.
3. Silence notifications.
Adolescents get 237 smartphone notifications a day, according to a 2023 study which surveyed 203 teens and tween between ages 11 and 17. Almost a quarter, 23%, arrived during school.
Silencing your notifications can help you stay present and productive during the hours it matters most.
Rausch emphasizes that getting rid of smartphones is not a panacea for depression. But, using your phone in a more thoughtful way can help you pursue activities that are proven to increase your happiness, like in-person social connection, and get more done.
“It’s not that we need to reject technology outright,” he says. “It’s that as technology is rapidly changing the way that we live our lives we need to press pause and think about how we want this to be in our lives. Is it fulfilling us? Is it helping us flourish? Is it helping us meet our goals? And, if not, what can we do to change it?”
Want to be a successful, confident communicator? Take CNBC’s new online course Become an Effective Communicator: Master Public Speaking. We’ll teach you how to speak clearly and confidently, calm your nerves, what to say and not say, and body language techniques to make a great first impression. Sign up today and use code EARLYBIRD for an introductory discount of 30% off through July 10, 2024.
Plus, sign up for CNBC Make It’s newsletter to get tips and tricks for success at work, with money and in life.
]]>According to NBC News report published this week by the Centers for Disease Control and Prevention revealed new details of the outbreak, which occurred in July 2022 at a nine-person family reunion in South Dakota.
One family member brought meat to the reunion from a black bear hunted in northern Canada. The meat had been frozen in a household freezer for 45 days. Hunting black bears is legal in Canada and many U.S. states.
The family made kebabs with the thawed meat, alongside grilled vegetables. According to the CDC, the family had a hard time determining whether the kebabs were fully cooked, because the meat was dark in color. So it was unintentionally served and eaten rare.
A week later, one family member — a 29-year-old man in Minnesota — developed a fever, severe muscle pain and swelling around the eyes. He was hospitalized twice for his symptoms.
The man tested positive for antibodies to Trichinella, a type of roundworm. Five other family members also developed symptoms such as fevers, headache, stomach pain, diarrhea, muscle pain and swelling around the eyes.
Two others who’d been exposed did not develop symptoms, and the CDC could not confirm whether the ninth person had been exposed to Trichinella.
The CDC tested the remaining frozen meat and detected larvae from the same roundworm species.
The agency presumed that all six family members had trichinellosis, a disease caused by eating undercooked meat contaminated with Trichinella larvae.
Such infections are rare. From January 2016 to December 2022, the CDC identified seven trichinellosis outbreaks in the U.S. involving 35 probable or confirmed cases. Most were linked to bear meat.
Trichinellosis is not the same parasitic infection that presidential candidate Robert F. Kennedy Jr. recently revealed he once suffered from. Kennedy said the brain infection he got comes from pork tapeworm larvae.
Two of the infected people at the family reunion ate the vegetables without the meat, the CDC said. Trichinella-infected meat can result in cross-contamination, so meat and its juices should be separated from other foods during cooking.
Three of the family members were hospitalized, each of whom had consumed the bear meat. They received a treatment called albendazole, which kills parasitic worms and their larvae.
All six people recovered from the disease.
The CDC report warns that freezing meat won’t kill all species of Trichinella. The bear meat at the family reunion, for instance, was contaminated with a species found in Arctic bears that’s resistant to freezing.
“Persons who consume meat from wild game animals should be aware that that adequate cooking is the only reliable way to kill Trichinella parasites,” the report’s authors wrote.
The CDC recommends cooking wild game meat to an internal temperature of at least 165 degrees Fahrenheit, which should be verified with a meat thermometer — not by looking at the color of the meat.
This story first appeared on NBCNews.com. More from NBC News:
]]>Nutritionists highly recommend starting your morning with foods that will keep you energized throughout the day like protein shakes and whole grain oats, experts told CNBC Make It last January. And cereal doesn’t fit that category.
“I never recommend cereal to my patients to have for breakfast, simply because it’s processed. I don’t recommend anything that’s processed,” said Dr. Nancy Rahnama, an internist and clinical nutritionist.
But cereal is a convenient choice if you’re short on time, Rahnama acknowledged. If you have only enough time to grab a bowl of cereal, reach for ones that are high in fiber and low in sugar, she advised.
Here is a list of cereals that are high in nutrients like fiber and protein and low in added sugar, according to a recent report by Healthnews.com.
Cereals with the most nutrients, lowest added sugar
Healthnews.com worked with nutrition scientist Lauryna Nelkine to compare the ingredients of the 15 most popular cereals in America, “taking into account their sales volumes and ratings on major American retailing platforms,” the report states.
These are the cereals that had the most nutrients, vitamins and minerals to support health, and the lowest amounts of added sugar.
- Highest in dietary fiber: Post Raisin Bran — 9 grams of fiber per serving
- Highest in protein: Special K Protein — 7 grams of protein per serving
- Highest in calcium and vitamin D: Multi Grain Cheerios — 200 milligrams of calcium and 4 micrograms of vitamin D
- Lowest in added sugar: Corn Flakes, Corn Chex and Special K Protein — 4 grams of sugar per serving each
Cereals with high levels of added sugar
Here are the cereals that stood out as having the highest amounts of added sugar (with 12 grams of added sugar per serving each):
- Lucky Charms
- Frosted Flakes
- Cinnamon Toast Crunch
- Froot Loops
- Reese’s Puffs
- Fruity Pebbles
Want to be a successful, confident communicator? Take CNBC’s new online course Become an Effective Communicator: Master Public Speaking. We’ll teach you how to speak clearly and confidently, calm your nerves, what to say and not say, and body language techniques to make a great first impression. Sign up today and use code EARLYBIRD for an introductory discount of 30% off through July 10, 2024.
Plus, sign up for CNBC Make It’s newsletter to get tips and tricks for success at work, with money and in life.
]]>As the lights dimmed in an operating room at The Mount Sinai Hospital in New York City, Dr. Joshua Bederson prepared to make history.
Bederson, system chair for the Department of Neurosurgery at Mount Sinai Health System, is no stranger to long hours in an operating room. The former competitive gymnast has completed more than 6,500 procedures in his career, and he said he visualizes the steps for each one as if he’s rehearsing for a routine.
On this particular morning in April, Bederson was readying for a meningioma resection case, which meant he would be removing a benign brain tumor. Bederson said his primary focus is always on caring for the patient, but in some cases, he also gets to help advance science.
This procedure was one such case.
A small crowd gathered as Bederson took his seat in the operating room, his silhouette aglow from the bright white light shining on the patient in front of him. Health-care workers, scientists and CNBC craned forward – some peering through windows – to watch as Bederson placed four electrode arrays from Precision Neuroscience onto the surface of the patient’s brain for the first time.
An electrode is a small sensor that can detect and carry an electrical signal, and an array is a grid of electrodes. Neurosurgeons use electrodes during some procedures to help monitor and avoid important parts of the brain, like areas that control speech and movement.
Precision is a three-year-old startup building a brain-computer interface, or a BCI. A BCI is a system that decodes neural signals and translates them into commands for external technologies. Perhaps the best-known company in the field is Neuralink, which is owned by Tesla and SpaceX CEO Elon Musk.
Other companies like Synchron and Paradromics have also developed BCI systems, though their goals and designs all vary. The first application of Precision’s system will be to help patients with severe paralysis restore functions like speech and movement, according to its website.

Precision’s flagship BCI is called the Layer 7 Cortical Interface. It’s a microelectrode array that’s thinner than a human hair, and it resembles a piece of yellow scotch tape. Each array is made up of 1,024 electrodes, and Precision says it can conform to the brain’s surface without damaging any tissue.
When Bederson used four of the company’s arrays during the surgery in April, he set a record for the highest number of electrodes to be placed on the brain in real-time, according to Precision. But perhaps more importantly, the arrays were able to detect signals from the patient’s individual fingers, which is a far greater amount of detail than standard electrodes are able to capture.
Using Precision’s electrode array is like turning a pixilated, low-resolution image into a 4K image, said Ignacio Saez, an associate professor of neuroscience, neurosurgery and neurology at the Icahn School of Medicine at Mount Sinai. Saez and his team oversee Precision’s work with Mount Sinai.
“Instead of having 10 electrodes, you’re giving me 1,000 electrodes,” Saez told CNBC in an interview. “The depth and the resolution and the detail that you’re going to get are completely different, even though they somehow reflect the same underlying neurological activity.”
Bederson said accessing this level of detail could help doctors be more delicate with their surgeries and other interventions in the future. For Precision, the ability to record and decode signals from individual fingers will be crucial as the company works to eventually help patients restore fine motor control.
The data marks a milestone for Precision, but there’s a long road ahead before it achieves some of its loftier goals. The company is still working toward approval from the U.S. Food and Drug Administration, and it has yet to implant a patient with a more permanent version of its technology.
“I think these are little baby steps towards the ultimate goal of brain-computer interface,” Bederson told CNBC in an interview.
Inside the operating room

Bederson’s surgery in April was not Precision’s first rodeo. In fact, it marked the 14th time that the company has placed its array on a human patient’s brain.
Precision has been partnering with academic medical centers and health systems to perform a series of first-in-human clinical studies. The goal of each study varies, and the company announced its collaboration with Mount Sinai in March.
At Mount Sinai, Precision is exploring different applications for its array in clinical settings, like how it can be used to help monitor the brain during surgery. In these procedures, surgeons like Bederson temporarily place Precision’s array onto patients who are already undergoing brain surgery for a medical reason.
Patients give their consent to participate beforehand.
It’s routine for neurosurgeons to map brain signals with electrodes during these types of procedures. Bederson said the current accepted practice is to use anywhere between four to almost 100 electrodes – a far cry from the 4,096 electrodes he was preparing to test.

Precision’s arrays are in use for a short portion of these surgeries, so CNBC joined the operating room in April once the procedure was already underway.
The patient, who asked to remain anonymous, was asleep. Bederson’s team had already removed part of their skull, which left an opening about the size of a credit card. Four of Precision’s arrays were carefully laid out on a table nearby.
Once the patient was stabilized, Precision’s employees trickled into the operating room. They helped affix the arrays in an arc around the opening on the patient’s head, and connected bundles of long blue wires at the other end to a cart full of equipment and monitors.
Dr. Benjamin Rapoport, Precision’s co-founder and chief scientific officer, quietly looked on. Every major procedure presents some risks, but the soft-spoken neurosurgeon’s calm demeanor never wavered. He told CNBC that each new case is just as exciting as the last, especially since the company is still learning.

Bederson entered the operating room as Precision’s preparations neared their end. He helped make some final tweaks to the set up, and the overhead lights in the operating room were turned off.
Ongoing chatter quieted to hushed whispers. Bederson was ready to get started.
He began by carefully pulling back a fibrous membrane called the dura to reveal the surface of the brain. He laid a standard strip of electrodes onto the tissue for a few minutes, and then it was time to test Precision’s technology.
Using a pair of yellow tweezers called long bayonet forceps, Bederson began placing all four of Precision’s electrode arrays onto the patient’s brain. He positioned the first two arrays with ease, but the last two proved slightly more challenging.
Bederson was working with a small section of brain tissue, which meant the arrays needed to be angled just right to lay flat. For reference, imagine arranging the ends of four separate tape measures within a surface area roughly the size of a rubber band. It took a little reconfiguring, but after a couple of minutes, Bederson made it happen.
Real-time renderings of the patient’s brain activity swept across Precision’s monitors in the operating room. All four arrays were working.
In an interview after the surgery, Bederson said it was “complicated” and “a little bit awkward” to place all four arrays at once. From a design perspective, he said two arrays with twice as many points of contact, or longer arrays with greater spacing would have been helpful.
Bederson compared the arrays to spaghetti, and the description was apt. From where CNBC was watching, it was hard to tell where one stopped and the next began.
Once all the arrays were placed and actively detecting signals, Precision’s Rapoport stood with his team by the monitors to help oversee data collection. He said the research is the product of a true team effort from the company, the health system and the patient, who often doesn’t get to see the benefits of the technology at this stage.
“It takes a village to make this sort of thing move forward,” Rapoport said.
CNBC left the operating room as Bederson began removing the tumor, but he said the case went well. The patient woke up afterward with some weakness in their foot since the surgery was within that part of the brain, but Bederson said he expected the foot would recover in around three to four weeks.

Rapoport was present at this particular surgery because of his role with Precision, but he’s well acquainted with the operating rooms at Mount Sinai.
Rapoport is a practicing surgeon and serves as an assistant professor of neurosurgery at the Icahn School of Medicine at Mount Sinai. Rapoport reports to Bederson, and Bederson said the pair have known one another since Rapoport was in residency at Weill Cornell Medicine.
Dr. Thomas Oxley, the CEO of the competing BCI company Synchron, is also a faculty member under Bederson. Synchron has built a stent-like BCI that can be inserted through a patient’s blood vessels. As of early February, the company had implanted its system into 10 human patients. It is also working toward FDA approval.
Bederson has an equity stake in Synchron, but he told CNBC he didn’t realize how much it would prevent him from participating in research with the Synchron team. He has no monetary investment in Precision.
“I really did not want to have any financial interest in Precision because I think it has an equally promising future and wanted to advance the science as fast as I could,” Bederson said.
Rapoport also helped co-found Musk’s Neuralink in 2017, though he departed the company the following year. Neuralink is building a BCI designed to be inserted directly into the brain tissue, and the company recently received approval to implant its second human patient, according to a report from The Wall Street Journal on Monday.
As the BCI industry heats up, Bederson said the amount that scientists understand about the brain is poised to “explode” over the next several years. Companies like Precision are just getting started.

“I really feel like the future is where the excitement is,” Bederson said.
Rapoport said Precision is hoping to receive FDA approval for the wired version of its system “within a few months.” This version, which is what CNBC saw in the operating room, would be for use in a hospital setting or monitored care unit for up to 30 days at a time, he said.
Precision’s permanent implant, which will transmit signals wirelessly, will go through a separate approval process with the FDA.
Rapoport said Precision hopes to implant “a few dozen” patients with the wired version of its technology by the end of the year. That data collection would give the company a “very high level of confidence” in its ability to decode movement and speech signals in real-time, he said.
“Within a few years, we’ll have a much more advanced version of the technology out,” Rapoport said.
]]>Sunscreen is a must-have for those outdoor activities under the sun. By reflecting or absorbing the sun’s ultraviolet (UV) rays, sunscreen can save you from developing a painful case of sunburn and help keep your skin healthy in the long run. Most skin cancers are caused by too much exposure to UV rays, according to the American Cancer Society.
While sunscreen doesn’t completely block all UV rays, it’s still an important tool in protecting your skin from sun damage.
So, how do you go about picking the right sunscreen? Here are five tips:
1. Use broad-spectrum sunscreen
Make sure your sunscreen is labeled as “broad spectrum,” which means it offers protection against both UVA and UVB rays.
Only broad-spectrum sunscreens with a sun protection factor (SPF) of 15 or higher can state that they protect against skin cancer and early skin aging, per the American Cancer Society.
2. Go with an SPF of 30 or higher
The American Cancer Society recommends using sunscreen with an SPF of at least 30. SPF denotes a sunscreen’s level of protection against UVB rays, which are the main cause of sunburn.
“When applying an SPF 30 sunscreen correctly, you get the equivalent of 1 minute of UVB rays for each 30 minutes you spend in the sun,” the American Cancer Society explains. “So, 1 hour in the sun wearing SPF 30 sunscreen is the same as spending 2 minutes totally unprotected.”
There are sunscreens with SPFs above 100, but it’s important to remember there isn’t a number that offers complete protection. The difference in protection level also becomes smaller as the SPF number increases. SPF 15 sunscreens filter out roughly 93% of UVB rays compared to roughly 97% with SPF 30, roughly 98% with SPF 50 and roughly 99% with SPF 100.
3. Pick a water-resistant sunscreen
The American Cancer Society says a sunscreen can’t be billed as “waterproof” or “sweatproof” because those are misleading terms. But there are sunscreens that are water resistant. Water-resistant sunscreens have to say whether they protect the skin for 40 or 80 minutes of swimming or sweating.
The CDC recommends applying a thick layer of sunscreen at least 20 minutes before going outside. Reapplying at least every two hours and each time you get out of the water or sweat heavily are other recommended practices.
4. Don’t just rely on other products that offer SPF protection
There are products like concealers and moisturizers that feature protection against UV rays. But Cleveland Clinic dermatologist Amy Kassouf still suggests using sunscreen, as other products with a built-in SPF may not offer enough protection.
“We put on just as much as we feel we can spread easily, then we’re off to the races. So, we don’t usually get the full protection listed on the label,” Kassouf said, via Health.ClevelandClinic.org.
“Apply sunscreen with at least SPF 30 and makeup with SPF 30. Together, they’ll add up to the protection you need.”
Kassouf advises to apply sunscreen first before other facial products.
5. Apply even if you have darker skin
The darkest skin tone provides only the equivalent of SPF 13, according to the Skin Cancer Foundation. So while those with darker skin may have more natural protection against UV rays compared to those with lighter skin, the risk for sun damage remains.
Sunscreen shouldn’t be the only way you protect your skin from the sun, either. The American Cancer Society also recommends seeking shade, covering your skin with clothing, wearing a hat and sporting sunglasses that block UV rays.
]]>“The last thing you want is for people to go home and remember the beach trip because they got sick,” Dr. John Torres, NBC News senior medical correspondent, said on TODAY.
Leaving food unrefrigerated for a prolonged period can cause certain bacteria, like E. coli and and salmonella, to grow and make those consuming the food sick with diarrhea and vomiting. Between 40 and 140 degrees Fahrenheit is known as the food “danger zone” for this reason, according to the U.S. Department of Agriculture.
Even if you use a cooler, you may not be able to completely prevent the temperature of your food from reaching above 40 degrees Fahrenheit. That’s why Torres recommends leaving certain items at home and choosing safer beach foods instead.
Foods not to bring to the beach
Cold cuts that require refrigeration
If you’re packing sandwiches made with deli meats or cold cuts — such as sliced turkey, ham, chicken, roast beef, salami, bacon or bologna — these need to be refrigerated until they are ready to eat, says Torres.
Although deli meats are often cured and processed to help prevent spoilage and contamination, they can still contain bacteria that can make you sick and multiple rapidly in warm temperatures, TODAY.com previously reported.
Keeping them in a cooler can make this food choice less risky, but once you take them out of the refrigerated environment, eat them right away.
Fresh salads
Fruits and vegetables contaminated with salmonella, E. coli, listeria and other bacteria are responsible for a large chunk of foodborne illness in the U.S., according to the U.S. Centers for Disease Control and Prevention.
Washing produce can reduce the amount but doesn’t entirely eliminate dangerous bacteria, which love to multiply in warm, most environments — like an airtight container holding salad on the beach.
Anything mayo-based
“Mayo-based potato salad is always one of those big things that cause a lot of issues,” says Torres.
Although it’s bought from a non-refrigerated shelf, mayonnaise does need to be refrigerated, and any salad or dish containing mayo can only be left out at temperatures above 40 degrees Fahrenheit for two hours before it should be discarded, according to the USDA.
Ideally, mayo-based foods should be refrigerated until they are served, says Torres. So it’s probably wise to avoid bringing these dishes to the beach entirely and enjoy them at home instead.
Raw meat
Grilling is a favorite beach activity for many. But bringing a bunch of raw hamburger meat or chicken that will sit around at various temperatures before it is barbecued is not the best idea due to the risk of bacteria growing. In general, Torres recommends against bringing raw or uncooked meats to the beach ever.
If you do decide to bring raw meat, store it properly in a cooler below 40 degrees Fahrenheit until it’s ready to be cooked, and keep it sealed and separate from any other foods that won’t get cooked in the cooler.
According to USDA guidelines, beef should be cooked to a minimum internal temperature of 145 degrees Fahrenheit, poultry to 165 degrees Fahrenheit and fish to 145 degrees Fahrenheit.
Precut fruits
Precut fruit is notorious for containing foodborne pathogens, such as salmonella. These include include papayas, peaches, and a major culprit, melon — including honeydew, cantaloupe and an all-time beach favorite, watermelon.
Cutting the fruit can transfer bacteria on the surface of the fruit into the flesh, where it can grow and multiply, especially in hot beach weather, TODAY.com previously reported.
Sliced fruit can also get warm faster and draw insects, says Torres. Instead of buying presliced fruit for your next beach trip, bring the whole thing and cut it up at the beach instead — just make sure you have clean hands and use a clean knife and cutting board.
Food safety tips for the beach
Many foods can be totally safe for the beach as long as you practice certain food safety precautions. Here’s some advice to keep in mind from Torres and the USDA:
- Perishable foods should never sit out of refrigeration for more than two hours, and if temperatures are 90 degrees are higher, no more than one hour.
- When packing, take food out of the fridge or freezer and put it immediately into the cooler. Aim to keep food refrigerated right up until you eat it.
- Try to avoid leftovers by only packing the amount you plan to consume.
- If you do choose to bring raw meat, season it at home, and keep it tightly wrapped in a place where any juices that may drip cannot get on to other foods, such as the bottom of the cooler.
- Pack drinks in a separate cooler from food so the food is exposed to warm temperatures less often.
- Try to fill your cooler all the way, packing extra ice if necessary, as this will keep its contents cool for longer.
- Bring a food thermometer if you are cooking at the beach.
This story first appeared on TODAY.com. More from Today:
]]>In a May 23 interview with Vogue, the singer, who now knows she has stiff person syndrome, recalled the early signs and symptoms of the rare neurological disorder.
It was 2008 and Dion was on her “Taking Chances World “Tour. “Quite rapidly, I was having difficulty controlling my voice,” Dion said. A visit to the otolaryngologist, or the ENT, found no abnormalities.
“They looked at (my vocal cords) from every angle, and they said it was pristine,” said Dion. So, she kept going. First, came a Las Vegas residency and then five more tours. During that time, she noticed her muscles stiffening and she needed support while walking.

More than 10 years later, with the onset of COVID in 2020, Dion, forced to slow down like the rest of the world, was afforded the time to finally get some answers. “When the pandemic arrived, I said to myself, the universe makes no mistakes, and I will take this time — this opportunity — to search,” Dion told Vogue.
After additional testing, doctors diagnosed Dion with the disorder that causes stiffness and spasms in the muscles for which there’s no cure. Symptoms of stiff person syndrome typically begin in the upper body, according to the Cleveland Clinic. As it progresses, symptoms typically move to the lower body, making it difficult to walk and increasing the risk of falling.
“It probably sounds very strange to say this to you, but when I was diagnosed, I was happy. I was finally able to move with the wave, not against it,” Dion said.
Since her diagnosis, Dion has been receiving treatment for her symptoms. “I haven’t beat the disease, as it’s still within me and always will be,” Dion told Vogue France in April. “Five days a week I undergo athletic, physical and vocal therapy. I work on my toes, my knees, my calves, my fingers, my singing, my voice,” she revealed.
At first, Dion says she questioned whether her illness was her fault. But, she said, there’s no way to know. “Life doesn’t give you any answers. You just have to live it!” So, she’s determined to find her way through life with SPS. Her chosen way forward is to “train like an athlete and work super hard,” she said. “I’ve chosen to work with all my body and soul, from head to toe, with a medical team. I want to be the best I can be. My goal is to see the Eiffel Tower again!”
Dion will give audiences an intimate look into her life with stiff person syndrome in a new documentary, “I Am: Celine Dion,” which will stream on Prime Video on June 25. It’s the first time she’s letting outsiders into her home.
“This is the only place I have that I could suffer, cry, go crazy, be happy, sing, miss a beat — and right now, to be vulnerable,” Dion told Vogue.
In the trailer for the film, Dion says, “I’m working hard every day, but I have to admit it’s been a struggle.” She talks about how much she misses singing, and how much she misses the stage and her fans. “If I can’t run, I’ll walk. If I can’t walk, I’ll crawl,” she says through tears. “I won’t stop.”
“I hope that the documentary doesn’t frighten people, but awakens people to SPS,” Dion told Vogue. “It took 17 years for me — please don’t wait that long.”
This story first appeared on TODAY.com. More from TODAY:
- Jason Kelce reacts to Harrison Butker speech, how he would feel if daughters were told to be homemakers
- Pat Sajak somehow kept it together during this hilarious and ‘spicy’ guess on ‘Wheel of Fortune’
- Céline Dion documentary trailer shows her tearing up as she struggles with stiff person syndrome
This story uses functionality that may not work in our app. Click here to open the story in your web browser.
]]>